Unlock Periodic Trends: Comprehensive Worksheet Answers Guide

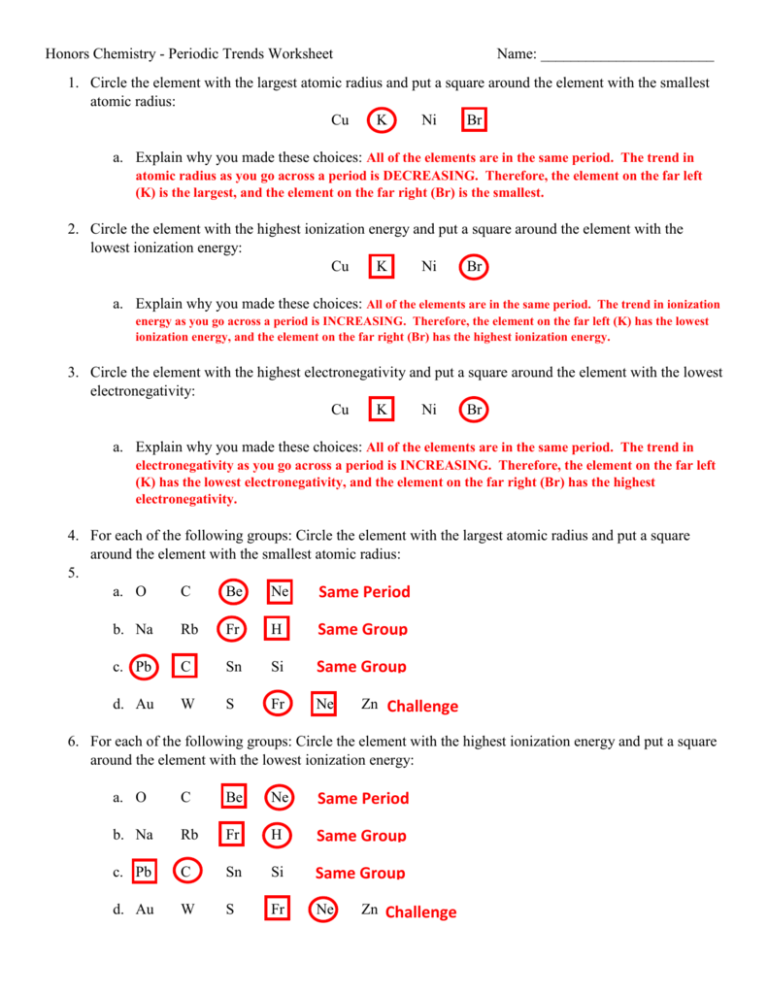
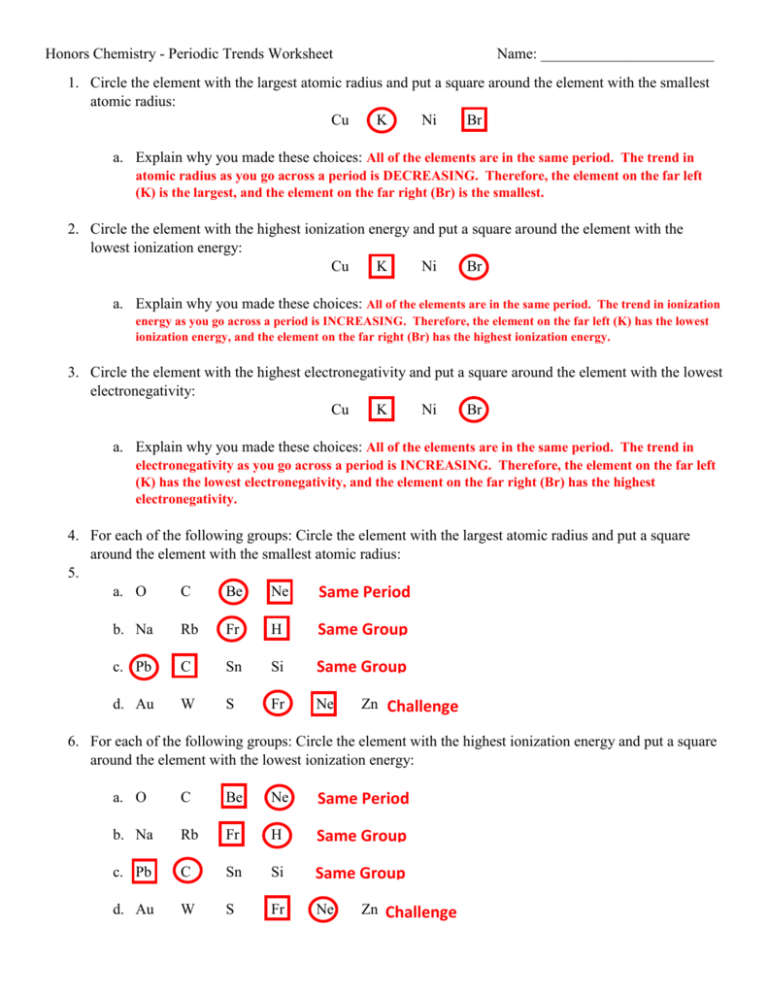
Understanding periodic trends in chemistry is pivotal for students and enthusiasts alike. The periodic table, often seen as a mere layout of elements, actually holds a treasure trove of patterns and trends that govern the behavior of elements. This guide aims to decode these trends through a series of commonly asked worksheet questions and provide comprehensive answers, fostering a deeper comprehension of the periodic table.
What are Periodic Trends?

Periodic trends refer to the predictable patterns in the physical and chemical properties of elements as you navigate the periodic table. These trends are influenced by atomic structure, electron configurations, and the way atoms interact with each other.
Atomic Radius

The atomic radius measures the size of an atom. Trends in atomic size can be understood as follows:
- Across a Period (from left to right): Atomic radii decrease. As you move across a period, the increasing nuclear charge pulls the electrons closer, reducing the atomic size.
- Down a Group: Atomic radii increase. With each new row, a new electron shell is added, which increases the distance of the outermost electrons from the nucleus, thereby increasing the atomic radius.

Ionization Energy
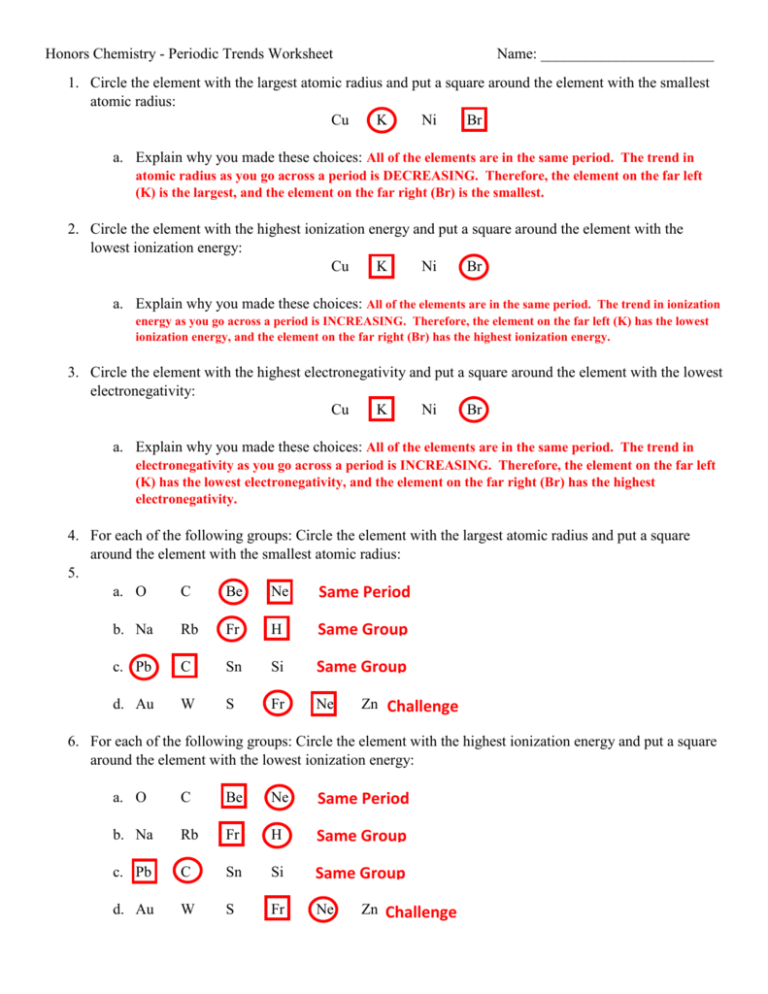
Ionization energy is the energy required to remove an electron from an atom or ion. Here’s how it behaves:
- Across a Period: Ionization energy generally increases. This increase is due to the enhanced nuclear attraction as the atomic size decreases.
- Down a Group: Ionization energy decreases. Electrons are further from the nucleus, requiring less energy to remove.
📌 Note: Noble gases, due to their full outer shells, have the highest ionization energies within their periods.
Electron Affinity

Electron affinity measures the energy change when an electron is added to an atom:
- Across a Period: It generally increases because smaller atoms attract an extra electron more easily.
- Down a Group: The trend is less consistent, but there’s a tendency to decrease due to the increased distance from the nucleus.
Electronegativity

Electronegativity indicates an atom’s ability to attract and hold onto electrons:
- Across a Period: Electronegativity increases from left to right because of the decreasing atomic size and increasing nuclear charge.
- Down a Group: Electronegativity decreases due to the larger atomic size and the increased shielding effect of inner electrons.

Worksheet Questions and Answers

Question 1: How does the atomic radius change across a period and down a group?

Across a period, atomic radius decreases due to increasing nuclear charge pulling electrons closer. Down a group, it increases because a new shell of electrons is added with each period.
Question 2: Why does ionization energy increase across a period but decrease down a group?

Ionization energy increases across a period because electrons are closer to the nucleus, making it harder to remove them. It decreases down a group as electrons are further from the nucleus and the shielding effect becomes more pronounced.
Question 3: Explain the trend in electron affinity across a period and down a group.

Electron affinity increases across a period due to smaller atomic size and the stronger pull of the nucleus. Down a group, the trend is less consistent, but generally, there is a slight decrease because electrons are further away, making the attraction less effective.
Question 4: How does electronegativity relate to ionization energy and electron affinity?

Electronegativity is related to both ionization energy and electron affinity. Higher electronegativity often means an element has a higher ionization energy (as electrons are strongly held) and may have a more positive electron affinity (indicating a tendency to gain electrons).
Summary
Periodic trends help predict the behavior of elements, from their physical sizes to how they interact with other atoms. By understanding these trends, one can navigate the periodic table with ease, understanding why elements behave the way they do in various chemical reactions and environments. This knowledge isn’t just academic; it has practical implications in fields like material science, biochemistry, and more.
What causes the trend in atomic radius down a group?

+
Down a group, atomic radius increases because each row introduces a new electron shell, increasing the distance from the nucleus to the outer electrons.
Why do noble gases have high ionization energies?
+
Noble gases have full electron shells, making it energetically unfavorable to remove an electron due to the stability of their electron configuration.
How can understanding periodic trends aid in chemical analysis?
+Understanding periodic trends allows chemists to predict reactivity, bond strength, and behavior in reactions, which can streamline analysis, synthesis, and material development.