Master Phase Change Calculations: Engaging Worksheet Guide
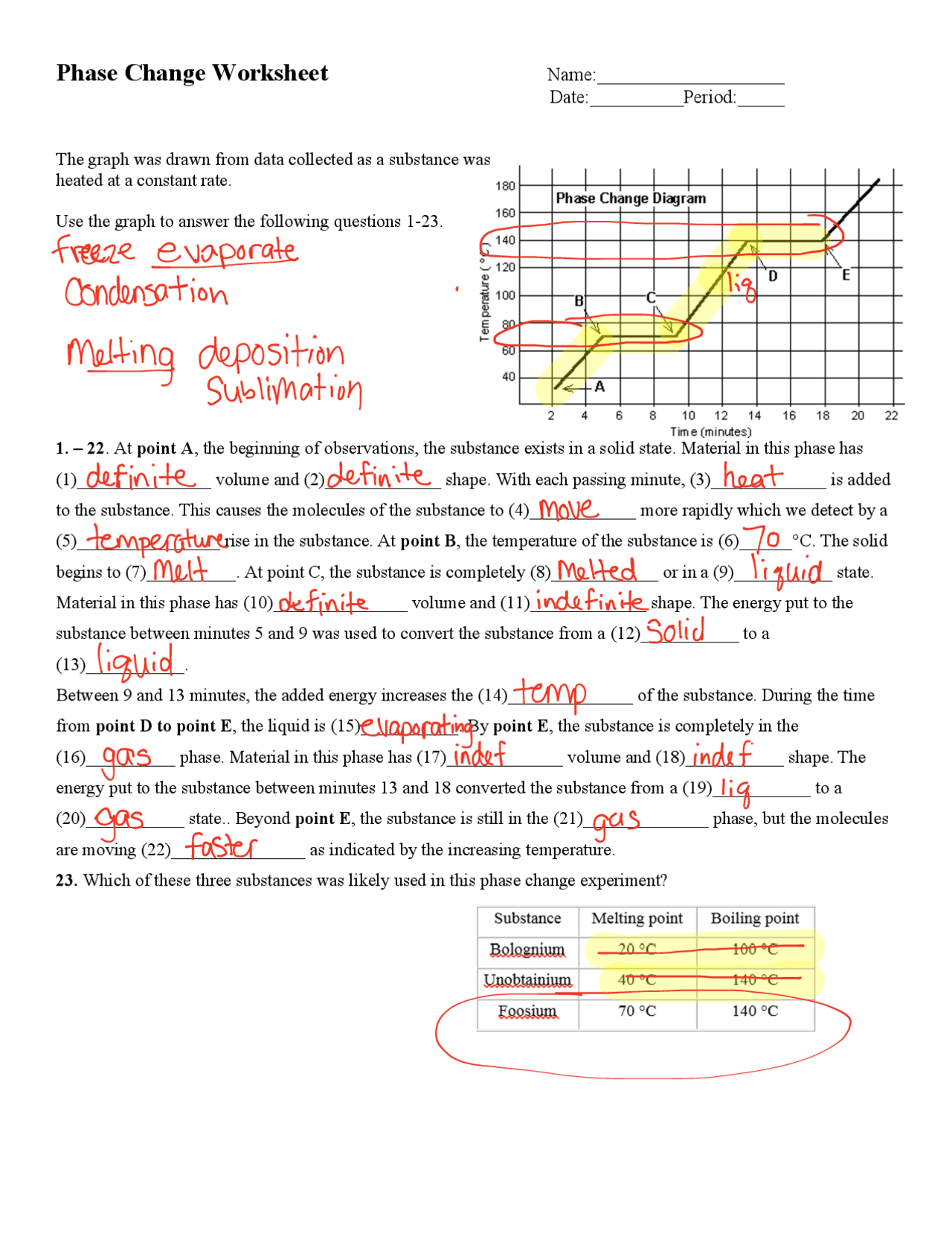
Understanding phase change calculations is fundamental for students and professionals in various scientific fields, including thermodynamics, physical chemistry, and material science. These calculations help predict how substances will behave under different temperature and pressure conditions, which is crucial for designing experiments, industrial processes, and understanding environmental phenomena. This comprehensive guide walks through key calculations related to phase changes, offering engaging worksheets and insights to enhance your learning experience.
Basic Concepts of Phase Changes

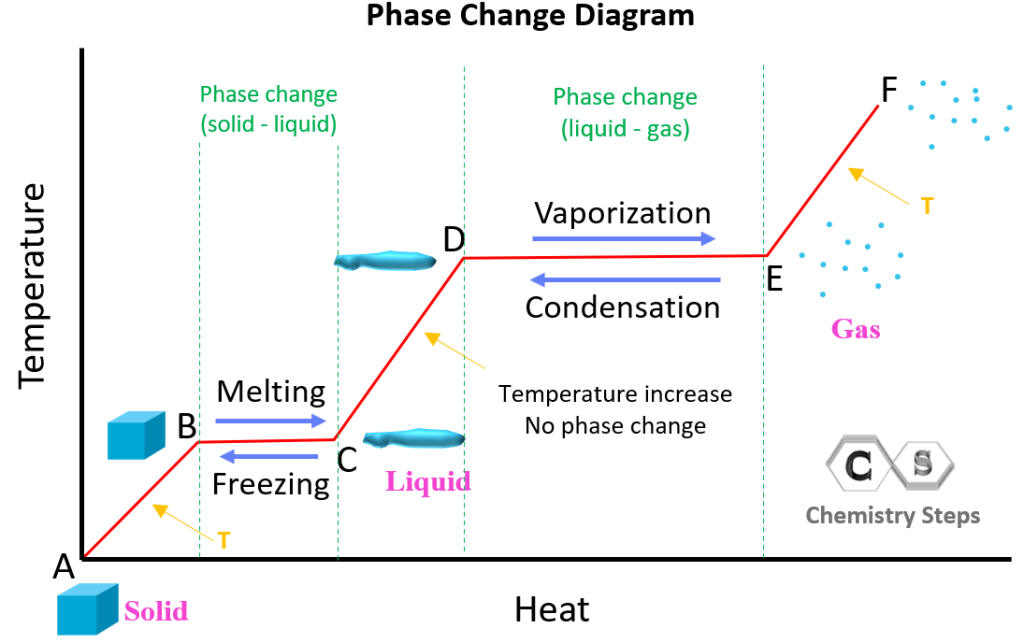
Phase Change: This refers to the transition of matter from one state to another, such as from solid to liquid, or liquid to gas. The primary types include melting (solid to liquid), freezing (liquid to solid), vaporization (liquid to gas), condensation (gas to liquid), sublimation (solid to gas), and deposition (gas to solid).
Heat of Fusion (ΔHfus): The amount of heat required to change one mole of a substance from a solid to a liquid without changing its temperature.
Heat of Vaporization (ΔHvap): The energy necessary to convert one mole of a substance from a liquid to a vapor state at the boiling point.
Heat Capacity (C): The amount of heat needed to raise the temperature of a substance by one degree Celsius.
Equations for Phase Changes

- Heat for Melting/Freezing: Q = m * ΔHfus, where Q is the heat, m is the mass, and ΔHfus is the heat of fusion.
- Heat for Vaporization/Condensation: Q = m * ΔHvap.
- Temperature Change: Q = m * C * ΔT, where ΔT is the change in temperature.
🔍 Note: Remember that these equations assume no heat is lost to the environment, which in real-world scenarios might not be the case.
Practical Application: Worksheet Exercises

Let's dive into some practical calculations through engaging worksheets:
Worksheet 1: Melting Ice
Calculate the heat required to melt 50 grams of ice at 0°C and heat it to 50°C. Use the following values: - Heat of fusion for ice: 334 J/g - Specific heat capacity of water: 4.184 J/g°C - Initial temperature of ice: 0°C - Final temperature of water: 50°C
| Step | Calculation |
|---|---|
| Heat for Melting | Qmelt = 50 g * 334 J/g = 16700 J |
| Heat for Temperature Increase | Qheat = 50 g * 4.184 J/g°C * 50°C = 10460 J |
| Total Heat Required | Total Q = Qmelt + Qheat = 27160 J |

⚙️ Note: In real-life applications, the efficiency of heating processes should be considered to account for losses in energy transfer.
Worksheet 2: Evaporating Water

Determine the total heat needed to convert 100 mL of water at 25°C to steam at 100°C. Assume the heat of vaporization of water at boiling point is 2260 J/g, and the density of water is 1 g/mL.
| Step | Calculation |
|---|---|
| Heat to Heat Water | Qheat = 100 g * 4.184 J/g°C * 75°C = 31380 J |
| Heat to Vaporize | Qvapor = 100 g * 2260 J/g = 226000 J |
| Total Heat Required | Total Q = Qheat + Qvapor = 257380 J |
Understanding Sublimation and Deposition

Sublimation and deposition are less common phase changes but are important for certain substances like dry ice or frost formation. Here are the calculations:
- Heat for Sublimation: Q = m * ΔHsub, where ΔHsub is the heat of sublimation.
- Heat for Deposition: Equal to the heat of sublimation but reversed.
Conclusion

By delving into phase change calculations, we not only understand how heat impacts the states of matter but also how these principles apply in real-world scenarios. From simple cooking to complex industrial processes, phase changes influence our daily lives in many ways. Using the equations and examples provided, you can now estimate the amount of heat needed for various phase transitions. This knowledge empowers you to make informed decisions in scientific and technical fields, optimizing processes and predicting outcomes with greater accuracy.
Why do we need to calculate phase changes?

+
Calculating phase changes is essential for predicting how substances behave under different temperature and pressure conditions, which is crucial for both scientific experiments and industrial applications.
What are some common errors in phase change calculations?

+
Common errors include incorrect use of units, neglecting to account for initial and final temperatures, or assuming ideal conditions without considering environmental factors like heat loss.
How does atmospheric pressure affect phase changes?

+
Atmospheric pressure can alter the boiling and melting points of substances. For example, at higher altitudes, where pressure is lower, water boils at a lower temperature than at sea level.
Can phase changes occur without changing temperature?

+
Yes, during processes like melting or freezing, a substance remains at its transition temperature until the phase change is complete, as all added or removed heat is used for changing the phase, not altering the temperature.