5 Ways to Ace Your Periodic Table Worksheet

If you're a chemistry student or an educator looking to master or teach the periodic table, worksheets can be an invaluable tool. Mastering the periodic table is crucial for understanding chemistry fundamentals. This guide will explore five essential strategies to excel at periodic table worksheets, ensuring that you or your students grasp the layout and secrets of the elements efficiently.
Understand the Structure of the Periodic Table
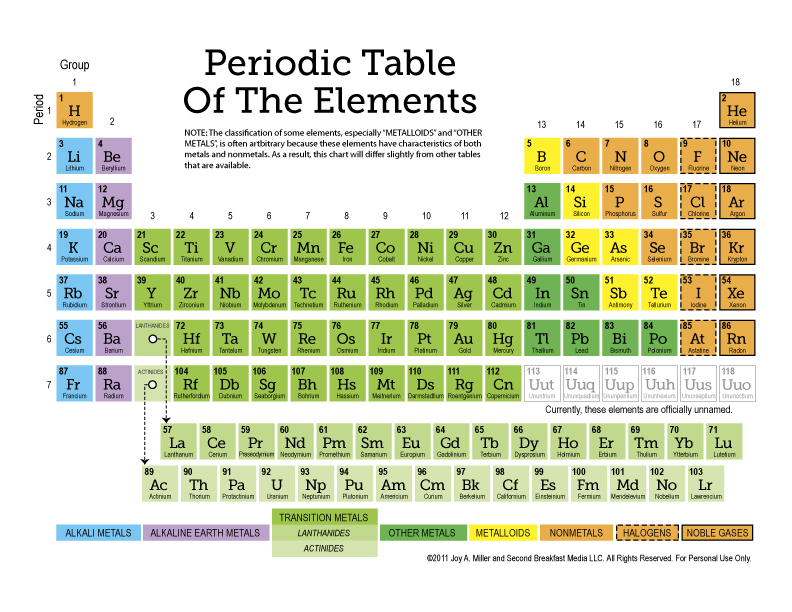
Before diving into any periodic table worksheet, it's critical to understand how the periodic table is organized:
- Rows (Periods): Elements in the same row have the same number of energy levels. From top to bottom, the periods represent an increase in atomic size.
- Columns (Groups or Families): Elements in the same column share similar chemical properties because of the same valence electron configuration. Key groups include:
- Group 1: Alkali Metals
- Group 2: Alkaline Earth Metals
- Group 17: Halogens
- Group 18: Noble Gases
- Blocks: The periodic table is divided into blocks that reflect the electron configuration:
- s-block (alkali and alkaline earth metals)
- p-block (includes metals, metalloids, and nonmetals)
- d-block (transition metals)
- f-block (lanthanides and actinides, often placed below the main body of the table)
🔍 Note: It's worth noting that the transition from one block to another often involves a change in electron shell or subshell filling.
Mastering Electron Configurations

Electron configurations can be a stumbling block on periodic table worksheets. Here’s how to tackle them:
- Aufbau Principle: Electrons occupy orbitals in order of increasing energy, filling the lowest energy levels first.
- Hund’s Rule: When filling orbitals of equal energy, electrons occupy empty orbitals singly before pairing up.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of quantum numbers; each orbital can hold a maximum of two electrons with opposite spins.
- Shell Diagrams: Use shell diagrams to visualize electron arrangements.
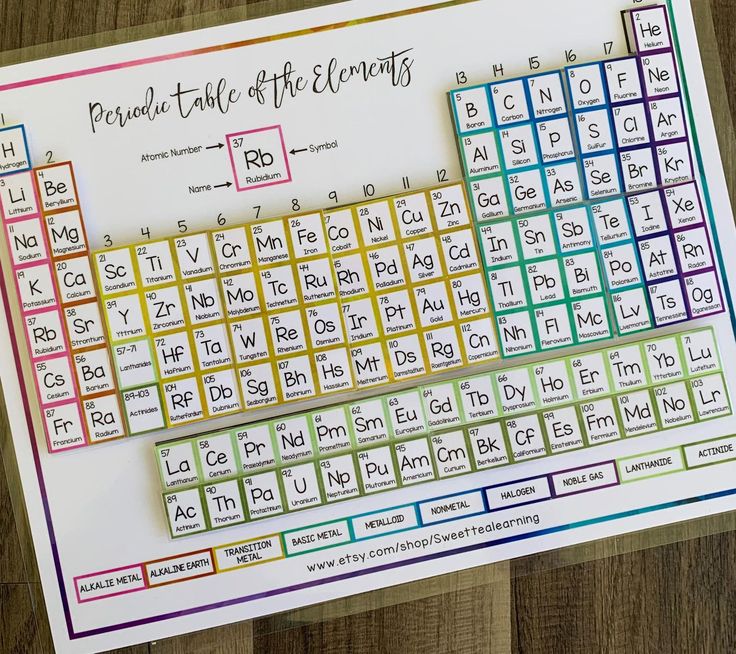
Identifying Trends and Patterns
The periodic table is rich with trends that can aid in answering worksheet questions:
| Trend | Description |
|---|---|
| Atomic Radius | Increases as you move down a group, decreases across a period. |
| Ionization Energy | Generally increases from left to right across a period, decreases down a group. |
| Electronegativity | Increases diagonally across periods (from bottom left to top right). |
| Metallic Character | Increases down a group, decreases across a period. |
| Valence Electrons | The number and arrangement of valence electrons largely determine an element’s chemical behavior. |

🔬 Note: Understanding these trends can help predict how elements will behave chemically, which is especially useful in reactivity questions.
Practice Identifying Elements by Properties

To excel in identifying elements:
- Learn element symbols: Start with the most common elements and gradually expand to lesser-known ones.
- Property Recognition: Memorize or understand the key properties (like luster, conductivity, density) of various element groups.
- Practice with Puzzles: Use crossword puzzles or fill-in-the-blank exercises that focus on elements’ characteristics.
- Flashcards: Create flashcards with element properties on one side and the element on the other to reinforce memory.
Formulate Logical Strategies for Worksheet Completion

When tackling a worksheet:
- Start with what you know: Fill in the blanks with elements and facts you are already confident about.
- Use Logical Deduction: For example, if you know the atomic number, you can determine the element’s location in the table and deduce its properties.
- Double Check: Use the trends and patterns to verify your answers. For instance, if you’re placing an element in the periodic table, check if its ionization energy matches the trend.
- Review Mistakes: Go over any incorrect answers to understand where you went wrong and learn from those mistakes.
In conclusion, by understanding the structure, mastering electron configurations, identifying trends, practicing element identification, and applying logical strategies, you're well on your way to acing any periodic table worksheet. Remember, chemistry is as much about understanding patterns and behaviors as it is about rote memorization. The key is to engage with the material actively, making connections between different aspects of the elements and their placement in the table.
What is the significance of periodic table groups?

+
The periodic table groups or families contain elements with similar chemical properties due to their similar electron configurations in the outermost shell. This grouping allows chemists to predict an element’s behavior in chemical reactions.
Why do atomic radii decrease across a period?
+
Atomic radii decrease across a period due to the increasing nuclear charge pulling the electrons closer to the nucleus, which results in a smaller atomic size.
How does understanding electron configurations help with chemistry?

+
Electron configurations give insights into how atoms will bond, how they react, and the energy levels involved in these processes. They are crucial for understanding both the stability and reactivity of elements.
Can you use the periodic table to predict bonding?

+
Yes, by knowing an element’s position, one can infer its valence electrons and bonding tendencies. Elements with fewer valence electrons tend to form positive ions (cations), while those with more valence electrons might gain electrons to form anions or share electrons in covalent bonds.
How often should I practice with periodic table worksheets?

+
Regular practice, ideally once or twice a week, can reinforce your understanding and memory of the periodic table. Remember to review and revise what you’ve learned periodically to maintain your knowledge.