Master Percent Yield: Fun Chemistry Problems & Solutions

Understanding Percent Yield in Chemical Reactions

Percent yield is a critical concept in chemistry that tells us how well a reaction works by comparing the actual amount of product obtained with the theoretical amount that should have been produced under ideal conditions. This measure helps chemists evaluate the efficiency of a chemical reaction, understand the practical limitations of reactions, and improve experimental techniques. This blog post aims to demystify percent yield, providing you with an understanding of its calculation, importance, and how it applies in real-world scenarios through fun chemistry problems and solutions.
What is Percent Yield?

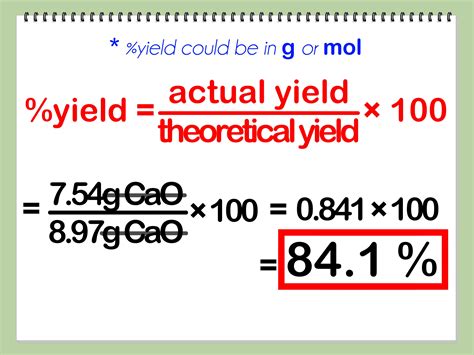
Percent yield is calculated using the formula:
[ \text{Percent Yield} = \left( \frac{\text{Actual Yield}}{\text{Theoretical Yield}} \right) \times 100 ]
- Actual Yield: This is the amount of product actually produced in a chemical reaction.
- Theoretical Yield: This is the maximum amount of product that could be produced from the reactants, based on stoichiometry or balanced chemical equations.
The percentage yield can tell you how close the experimental yield was to the calculated or expected yield, highlighting inefficiencies or losses in the process.
The Importance of Percent Yield

Understanding percent yield is not just an academic exercise; it has practical implications:
- Efficiency of Reaction: It indicates how well the reaction has gone. A low percent yield might suggest that side reactions occurred, there were errors in measuring the reactants, or the reaction conditions were not optimal.
- Cost-Effectiveness: In industrial settings, maximizing yield is crucial for cost-efficiency. Lower yields mean more waste, higher costs, and environmental impacts.
- Purification and Purity: If the percent yield is low, it might suggest impurities, or the need for additional purification steps which can be costly.
- Understanding Reaction Dynamics: Percent yield helps in studying reaction kinetics, catalysis, and understanding the mechanism of reactions.
Calculating Percent Yield: A Step-by-Step Guide
Here's how you can calculate the percent yield:
- Write down the balanced chemical equation: This step ensures you know the stoichiometry of the reaction.
- Determine the moles of the limiting reactant: This is the reactant that runs out first, determining the theoretical yield.
- Calculate the theoretical yield: Use stoichiometry to find out how much product should form from the moles of the limiting reactant.
- Measure the actual yield: Conduct the experiment and measure the amount of product formed.
- Calculate the percent yield: Plug in your actual and theoretical yields into the formula provided above.
Practical Example: Synthesis of Water

Consider the synthesis of water from hydrogen and oxygen:
[ 2H_2 + O_2 \rightarrow 2H_2O ]
If you start with 2 moles of H2 and an excess of O2, here's how you would calculate the percent yield:
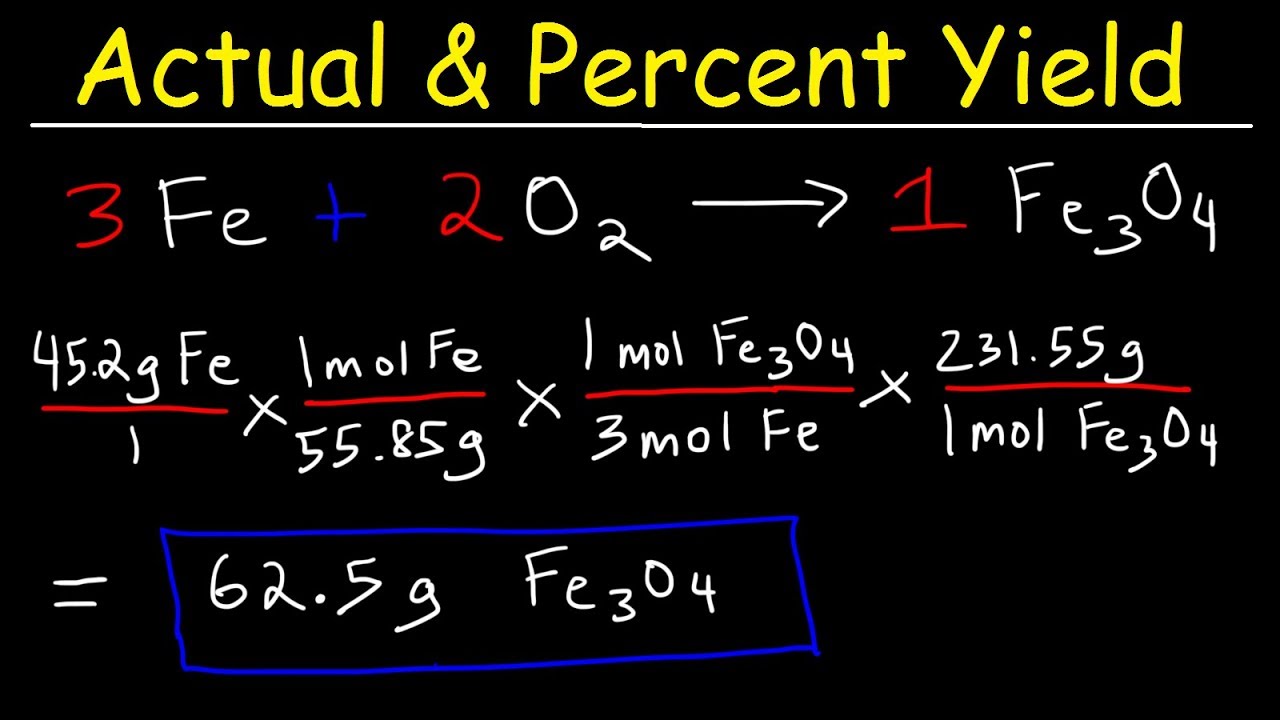
- Theoretical Yield: The reaction produces 2 moles of H2O for every 2 moles of H2, which is 2 x 18.0153 g/mol = 36.0306 grams of H2O.
- Actual Yield: Suppose the reaction gives you only 32.428 grams of H2O.
- Percent Yield: \[ \text{Percent Yield} = \left( \frac{32.428}{36.0306} \right) \times 100 = 90.00\% \]
Solutions to Common Percent Yield Problems

Let's address some common questions or problems students might face:
Scenario 1: When to Use Percent Yield?

Percent yield is useful in:
- Determining the efficiency of lab reactions.
- Evaluating industrial processes for production.
- Understanding potential sources of loss in reactions.
Scenario 2: Factors Affecting Percent Yield
Here are several reasons why you might not get a 100% yield:
- Incomplete Reaction: Not all reactants might react due to kinetic limitations or suboptimal conditions.
- Side Reactions: Sometimes, unintended reactions produce byproducts.
- Physical Losses: Product might stick to glassware or be lost during transfers.
- Purification Losses: Steps like filtration, evaporation, or recrystallization can lead to some loss of product.
- Measurement Errors: Incorrect measurement of reactants or product can lead to calculation errors.
🔬 Note: Always consider experimental conditions when troubleshooting low percent yields.
Real-World Applications of Percent Yield
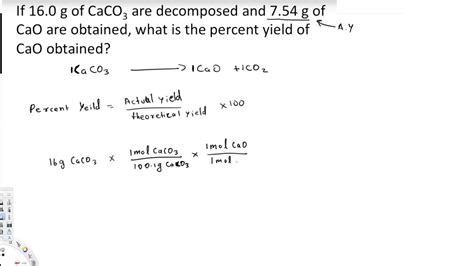
Percent yield isn't just a theoretical calculation. Here's how it's applied:
- Pharmaceutical Industry: High percent yields are crucial for cost-effectiveness and drug availability.
- Materials Science: Synthesis of new materials often requires optimizing reactions to increase percent yield.
- Environmental Chemistry: In remediation efforts, efficient use of reagents means less environmental impact.
- Food Science: In cooking or baking, understanding yield can affect the amount and quality of the final product.
Wrapping Up

Understanding and mastering percent yield calculation offers a window into the reality of chemical reactions beyond the balanced equations. By learning how to calculate, analyze, and interpret percent yields, you can diagnose why a reaction might not be working as expected and find ways to optimize processes. The key takeaways include:
- The formula for calculating percent yield involves comparing actual and theoretical yields.
- Percent yield provides insights into reaction efficiency, cost, and purification needs.
- Practical examples and real-world applications highlight the importance of this concept in various fields.
- Common problems with percent yield include physical losses, incomplete reactions, side reactions, and measurement errors.
Why is percent yield never 100%?

+
Percent yield is rarely 100% due to various real-world limitations such as side reactions, incomplete reactions, physical losses during transfer, and errors in measurement.
Can percent yield be over 100%?

+
Percent yield over 100% suggests an error in measurement or calculation. The actual yield cannot exceed the theoretical yield in an ideal system, as it would imply creating matter.
How can I improve percent yield?

+
To improve percent yield, consider optimizing reaction conditions, reducing side reactions, minimizing physical losses, and ensuring accurate measurements.