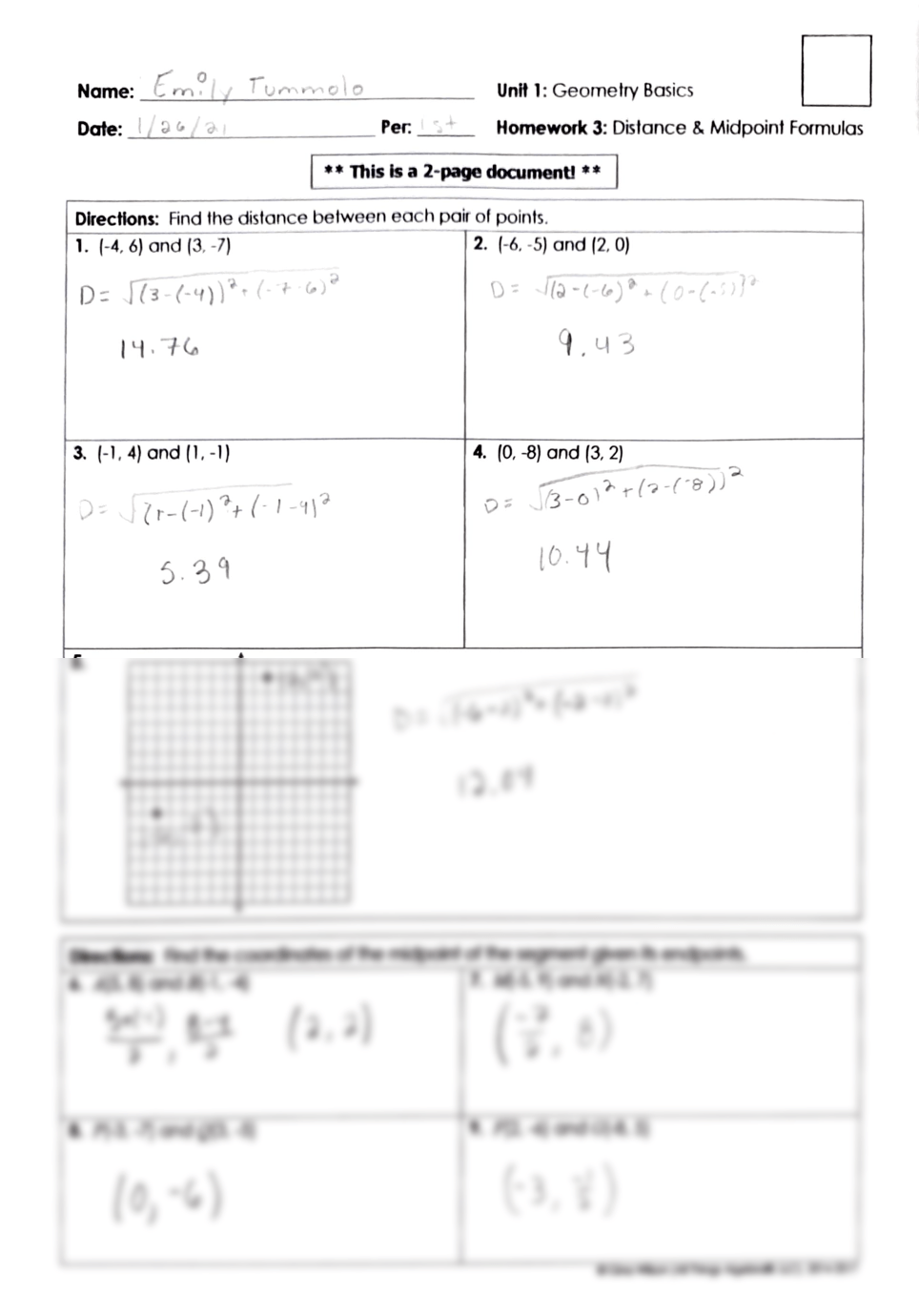
Mastering Oxidation-Reduction Reactions with Our Worksheet

Have you ever wondered how certain chemical reactions transfer electrons from one species to another? If you are preparing for a chemistry class or simply need to revisit the fundamentals, understanding oxidation-reduction reactions, often shortened to redox reactions, is crucial. Our redox reaction worksheet is an excellent tool for mastering this essential concept. Here’s how you can leverage it to enhance your understanding and ace your exams.
Understanding Redox Reactions

Redox reactions involve the transfer of electrons between chemical species, with one species losing electrons (oxidation) and another gaining them (reduction). These reactions are everywhere, from the rusting of iron to the process of cellular respiration. Here’s a basic breakdown:
- Oxidation: Loss of electrons; increase in oxidation state.
- Reduction: Gain of electrons; decrease in oxidation state.
- Oxidizing agent: The species that is reduced.
- Reducing agent: The species that is oxidized.
Identifying Redox Reactions

To identify if a reaction is redox, you need to:
- Check the oxidation states of all elements before and after the reaction.
- Look for changes in oxidation states.
- Confirm if there is an electron transfer involved.
Using Our Redox Reaction Worksheet

Our redox reaction worksheet provides a systematic approach to mastering redox reactions. Here’s how you can use it:
Step 1: Assigning Oxidation States

Before solving redox equations, you must determine the oxidation states:
- Free elements always have an oxidation state of 0.
- Hydrogen usually has +1, except in metal hydrides where it’s -1.
- Oxygen generally has -2, but -1 in peroxides and +2 in superoxides.
- The sum of oxidation states in a compound must equal its charge.
🔍 Note: It’s crucial to memorize these rules for efficiency in assigning oxidation states.
Step 2: Balancing Redox Reactions

Balancing redox reactions can be approached in two ways:
- Half-reaction method: Split the reaction into oxidation and reduction half-reactions, balance atoms and charges separately, and then combine them.
- Oxidation number method: Track changes in oxidation states directly to balance the equation.
Here’s how you might balance the reaction:
| Reactants | Oxidation States | Products | Oxidation States |
|---|---|---|---|
| CuO | Cu2+ | Cu | Cu0 |
| HCl | H+1, Cl-1 | H2O | H+1, O-2 |
| Cl2 | Cl0 |

By following the steps, you would find that the balanced equation is:
CuO + 2HCl → Cu + H2O + Cl2
Step 3: Solving Redox Problems

Our worksheet includes problems where you:
- Identify oxidizing and reducing agents.
- Calculate changes in oxidation state.
- Balance complex redox reactions under acidic or basic conditions.
Applying Redox Reactions in Real Life

Understanding redox reactions isn’t just academic; here are a few applications:
- Corrosion: Rusting of metals, where iron loses electrons.
- Batteries: Electron transfer drives electrical energy production.
- Photosynthesis: Conversion of light energy into chemical energy through electron transfer.
- Disinfection: Use of oxidizing agents like chlorine to kill bacteria.
⚠️ Note: Remember that redox reactions are not just about chemical changes but also play a vital role in energy processes and environmental chemistry.
Enhancing Comprehension and Memory

Here are some tips to effectively use our worksheet:
- Visualize electron flow by drawing arrow diagrams.
- Practice with real-life examples for better understanding.
- Use mnemonic devices like “LEO the lion says GER” (Loss of Electrons is Oxidation, Gain of Electrons is Reduction).
- Review and do self-assessment quizzes included in the worksheet.
With regular practice using our redox reaction worksheet, you'll not only master the concept but also gain confidence in tackling complex chemical reactions.
What is the difference between oxidation and reduction?

+
Oxidation is the loss of electrons or an increase in oxidation state, whereas reduction is the gain of electrons or a decrease in oxidation state.
How do I know if a reaction is redox?

+
Check for changes in oxidation states of elements involved. If there is an increase for some atoms and a decrease for others, it’s a redox reaction.
Why does balancing redox reactions require special methods?

+
Redox reactions involve electron transfer, which needs to be accounted for in the balancing process, hence the need for methods like the half-reaction or oxidation number approach.