Easy Oxidation Numbers Worksheet for Quick Learning
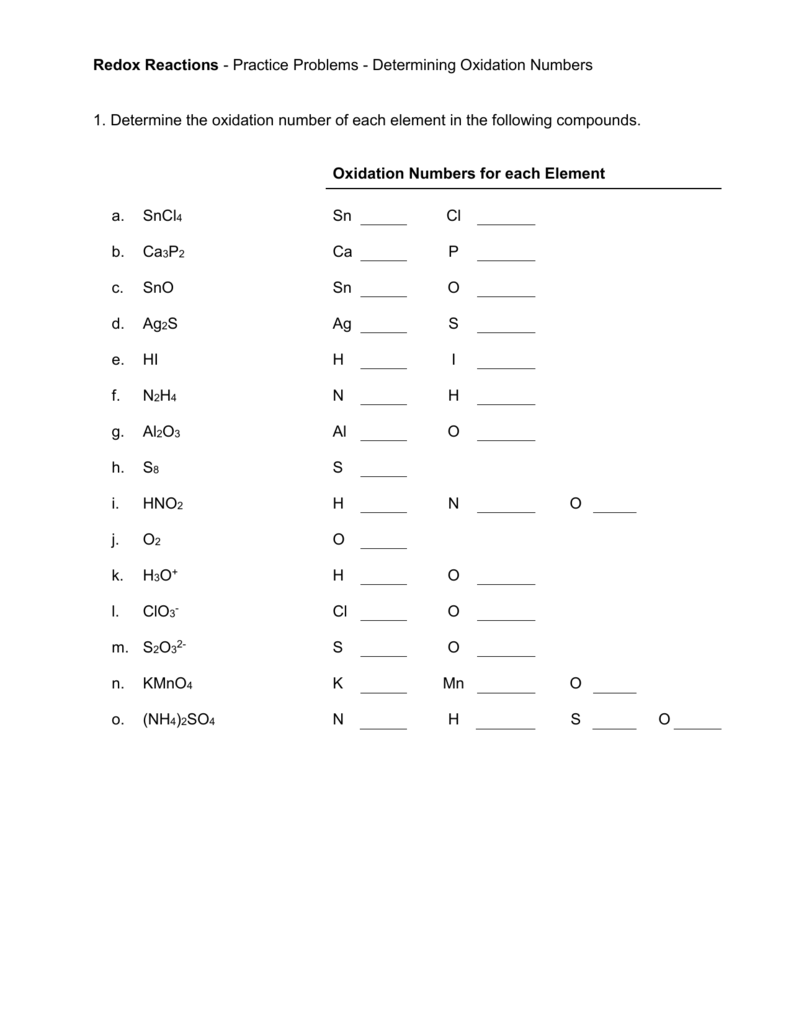
In the study of chemistry, understanding how atoms bond and interact with one another is crucial. One of the fundamental concepts in this arena is oxidation numbers, which helps us determine how electrons are distributed in compounds. Learning oxidation numbers isn't just a theoretical exercise; it's essential for understanding redox reactions, predicting chemical behavior, and even in applications like balancing chemical equations. This worksheet will guide you through an easy, step-by-step approach to mastering oxidation numbers.
Why Oxidation Numbers Matter

Before we dive into the worksheet, let’s briefly discuss why oxidation numbers are important:
- Redox Reactions: They are central to understanding oxidation and reduction processes.
- Chemical Nomenclature: Helps in naming compounds correctly.
- Predictive Power: Understanding oxidation states allows predictions of compound stability, reactivity, and electronic structure.
Assigning Oxidation Numbers
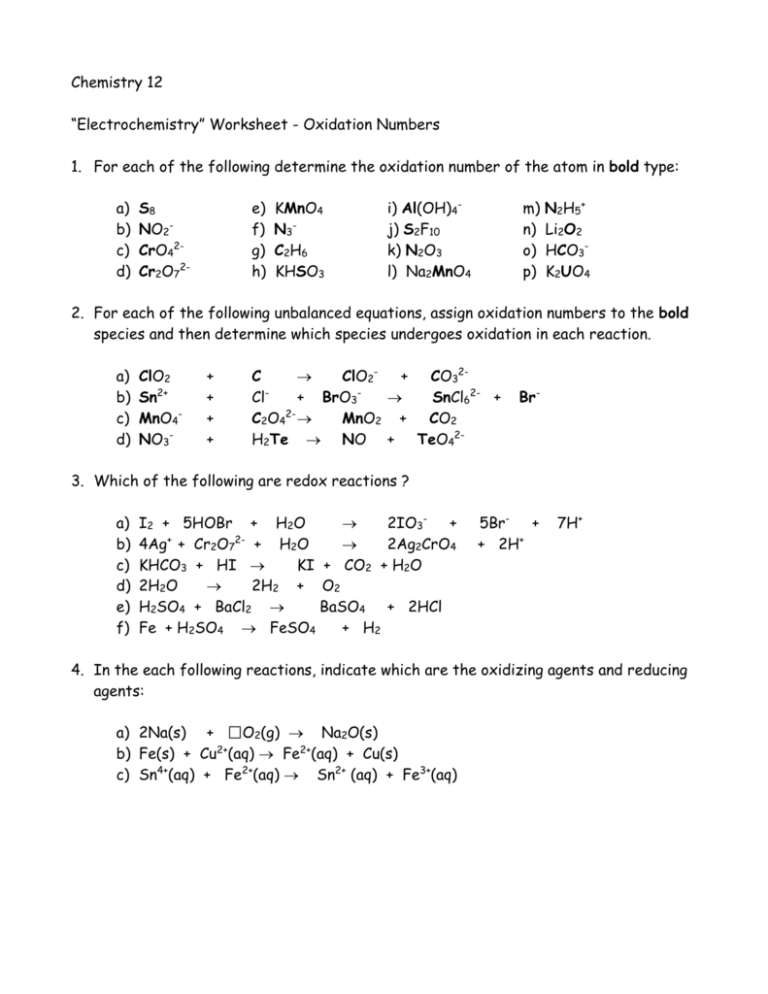
Here are the basic rules for assigning oxidation numbers:
- Element in its Free or Uncombined State: Its oxidation number is always zero, e.g., O₂, N₂.
- Monatomic Ions: The oxidation number of a monatomic ion is equal to its charge, e.g., Na⁺ = +1, Cl⁻ = -1.
- Combined Oxygen: Usually has an oxidation number of -2, except in peroxides (e.g., H₂O₂) where it’s -1.
- Combined Hydrogen: Typically has an oxidation number of +1 in compounds with nonmetals and -1 with metals, e.g., H₂O vs. NaH.
- Fluorine: Always -1 when bonded to other atoms.
- The Sum of Oxidation Numbers: Must be zero for neutral compounds and equal to the ion charge for polyatomic ions.
With these rules in mind, let’s get to the practical part:
Worksheet: Finding Oxidation Numbers

Below is a table that will help you practice assigning oxidation numbers to different atoms in various compounds:
| Compound | Element | Oxidation Number |
|---|---|---|
| H₂O | H | +1 |
| H₂O | O | -2 |
| NaCl | Na | +1 |
| NaCl | Cl | -1 |
| SO₄²⁻ | S | +6 |
| SO₄²⁻ | O | -2 |
| CO₂ | C | +4 |
| CO₂ | O | -2 |
| Fe₂O₃ | Fe | +3 |
| Fe₂O₃ | O | -2 |

Practical Tips for Learning

- Start with Simple Compounds: Begin with binary compounds or those you are familiar with.
- Memorize Common Ions: Knowing the oxidation numbers of common elements in their typical compounds can speed up your learning.
- Practice Regularly: Consistent practice helps in internalizing the rules and patterns.
Here are a few important notes to keep in mind while you're learning:
⚠️ Note: Always remember that oxidation numbers are assigned to atoms or ions and are not always real charges but rather a bookkeeping method.
Advanced Concepts

Once you’ve mastered the basics, you can explore:
- Polyatomic Ions: Assigning oxidation numbers in ions like SO₄²⁻, CO₃²⁻, and NO₃⁻.
- Coordination Compounds: These can have multiple oxidation states, making them more complex but also fascinating.
📚 Note: Coordination compounds often require more than just simple oxidation number rules; understanding their chemistry involves coordination number and ligand properties.
Closing Thoughts

Learning oxidation numbers is like unlocking a code in chemistry that allows you to predict, understand, and interact with chemical reactions more deeply. This worksheet provides you with the tools to master this concept through practice and understanding of the basic principles. Remember, consistent practice, understanding the rules, and applying them to real-world scenarios will solidify your knowledge. As you progress in your study, you’ll find oxidation numbers becoming an indispensable part of your chemical toolkit, making complex reactions more predictable and understandable.
Why are oxidation numbers important in chemistry?

+
They help us understand how electrons are distributed in compounds, which is essential for understanding redox reactions, chemical nomenclature, and predicting compound behavior.
Can an element have more than one oxidation number?

+
Yes, elements like transition metals often exhibit multiple oxidation states due to their ability to lose different numbers of electrons from their outer shells.
How can I quickly determine oxidation numbers?

+
Memorize common oxidation states for elements in familiar compounds, use the rules for assigning oxidation numbers, and practice regularly to make the process intuitive.