Mastering Osmosis: Your Ultimate Worksheet Answer Guide
Osmosis plays a fundamental role in biology, affecting everything from plant hydration to human physiology. Understanding osmosis through engaging with worksheets can solidify your grasp of this essential scientific concept. This guide provides a thorough walkthrough of osmosis worksheet answers, explaining the "how" and "why" behind each answer to enhance your learning experience.
The Basics of Osmosis

Osmosis is the movement of water molecules across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. Here are the key points:
- Semipermeable Membrane: A barrier that allows the passage of water but restricts larger solute molecules.
- Solute: The substance dissolved in a solution; in osmosis, this is usually something other than water.
- Solvent: The liquid, usually water, that dissolves the solute.
Table: Types of Osmotic Solutions

| Solution Type | Description | Cell Behavior |
|---|---|---|
| Isotonic | The concentrations of solutes inside and outside the cell are equal. | The cell remains in balance with no net water movement. |
| Hypertonic | The concentration of solutes outside the cell is higher than inside. | The cell loses water, potentially causing shrinkage or plasmolysis in plants. |
| Hypotonic | The concentration of solutes inside the cell is higher than outside. | The cell takes in water, potentially leading to swelling or bursting in animal cells, or turgidity in plant cells. |

Osmosis Worksheet Answers

Let’s delve into the common questions and answers you might encounter in an osmosis worksheet:
1. Defining Osmosis

Question: What is osmosis?
Answer: Osmosis is the process where water molecules move from an area of higher water potential (lower solute concentration) to an area of lower water potential (higher solute concentration) through a semipermeable membrane. This movement aims to balance the solute concentration on both sides of the membrane.
2. The Role of Membranes in Osmosis

Question: How do cell membranes play a part in osmosis?
Answer: Cell membranes are selectively permeable; they control what enters and exits the cell. In osmosis:
- Water can pass through the membrane via aquaporins or diffusion.
- Solutes, depending on their size and charge, might be blocked or allowed through channels or by diffusion.
3. Osmotic Pressure

Question: What is osmotic pressure?
Answer: Osmotic pressure is the pressure required to prevent osmosis from happening. It’s influenced by:
- The concentration of solutes on either side of the membrane.
- The temperature, which affects the rate of osmosis.
- The permeability of the membrane, which can change with physiological conditions.
💡 Note: Osmotic pressure is not a physical pressure applied to the solution, but rather a theoretical pressure that would prevent the movement of water molecules through the membrane.
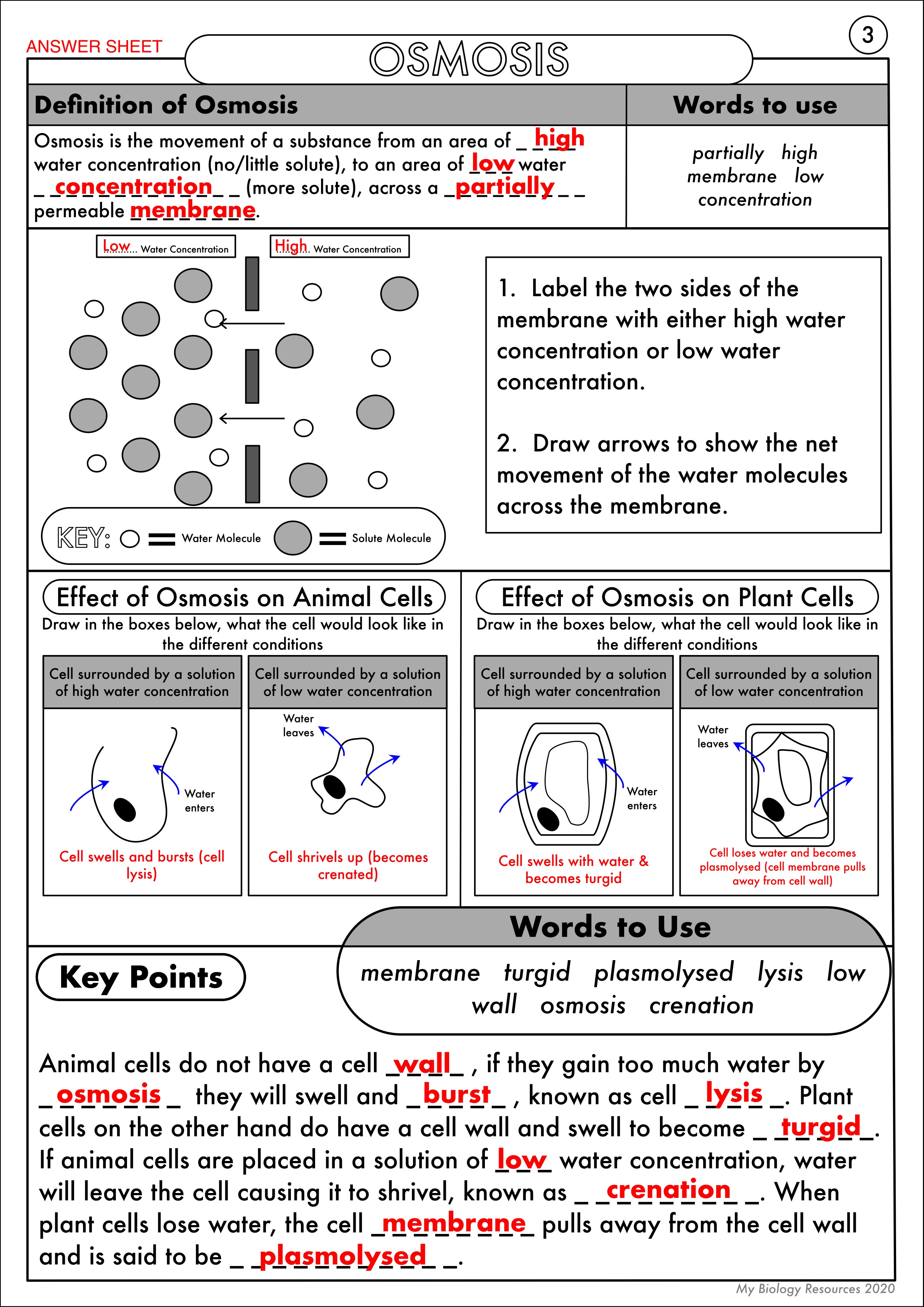
4. Effects of Osmosis on Plant and Animal Cells

Question: What happens to plant and animal cells in different solutions?
Answer: Here’s how different types of solutions impact cells:
- Isotonic Solution:
- Animal cells: Remain unaffected, no net water movement.
- Plant cells: Maintain turgidity with no net water movement.
- Hypertonic Solution:
- Animal cells: Shrink or shrivel due to water loss, leading to crenation.
- Plant cells: Lose water, plasmolysis occurs, and the cell wall prevents further shrinkage.
- Hypotonic Solution:
- Animal cells: Swell, potentially leading to lysis if the cell bursts.
- Plant cells: Become turgid, increasing in size but held in shape by the cell wall.
Applications in Real Life

Osmosis isn’t just a theoretical concept; it has practical applications:
- Water Purification: Reverse osmosis is used to desalinate water by forcing water through a semipermeable membrane against the osmotic gradient.
- Medicine: Osmosis affects how drugs are absorbed, the movement of fluids in the body, and the treatment of dehydration or overhydration.
- Food Preservation: Osmotic dehydration is used to preserve fruits by reducing water content, thus inhibiting microbial growth.
Notes on Laboratory Experiments

🔬 Note: Laboratory experiments with osmosis often use dyes, sucrose solutions, or potato strips to visualize osmotic effects. Be sure to follow safety protocols when handling chemicals or biological materials.
In this comprehensive guide to osmosis worksheet answers, we’ve explored the mechanics, applications, and theoretical implications of osmosis. By understanding these principles, you can better comprehend the multitude of biological processes where osmosis plays a pivotal role. Whether you’re studying for an exam, preparing for a lab, or simply curious about the science behind water movement in living organisms, this guide should serve as an invaluable resource to ensure you grasp the essential details and nuances of osmosis.
What is the difference between osmosis and diffusion?

+
Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration. Osmosis is a type of diffusion that specifically involves the movement of water molecules through a semipermeable membrane. The key difference lies in the involvement of a membrane in osmosis.
Can osmosis happen in both directions?

+
Yes, osmosis can happen in both directions depending on the concentration gradient. If the solute concentration on one side of the membrane increases, water will move in that direction. However, if the concentrations are equal (isotonic conditions), there’s no net movement of water.
How can we observe osmosis in daily life?

+
One common example is the swelling of dried fruits or vegetables when soaked in water. Plants also exhibit osmosis through their root systems by taking up water from the soil. In cooking, salt (solute) draws water out of vegetables, a process known as osmotic dehydration.

