Organic Compounds Worksheet: Master Chemistry Basics Easily

Understanding Organic Compounds
Organic compounds are a fascinating and integral part of chemistry, primarily composed of carbon bonded with hydrogen, oxygen, and other elements. Understanding these compounds can unlock a world of knowledge from everyday life applications like plastics and fuels to complex biochemical processes within living organisms. In this extensive blog post, we'll guide you through the basics of organic compounds, helping you master the fundamental concepts with ease.
What Are Organic Compounds?

Organic compounds are those that are based on carbon atoms bonded covalently to one another and other elements such as:
- Hydrogen (H)
- Oxygen (O)
- Nitrogen (N)
- Phosphorus (P)
- Sulfur (S)
- and Halogens (F, Cl, Br, I)
Carbon atoms can bond with each other in a variety of structures, including chains, branches, or rings, forming a rich variety of compounds. The uniqueness of carbon lies in its ability to form stable bonds with itself and other elements, leading to a vast array of molecular structures.
Key Classes of Organic Compounds

Let's delve into the fundamental classes of organic compounds:
Alkanes

Alkanes are saturated hydrocarbons with all single bonds between carbon atoms. They are also known as paraffins. Here are some characteristics:
- General Formula: CnH2n+2
- Structure: Linear or branched chains.
- Example: Methane (CH4), Ethane (C2H6)

Alkenes

These compounds contain at least one double bond between carbon atoms, making them unsaturated hydrocarbons:
- General Formula: CnH2n
- Structure: Acyclic or cyclic with one or more double bonds.
- Example: Ethene (C2H4)
Alkynes

Alkynes are hydrocarbons with at least one triple bond between carbon atoms:
- General Formula: CnH2n-2
- Structure: Usually linear with at least one triple bond.
- Example: Ethyne (C2H2)
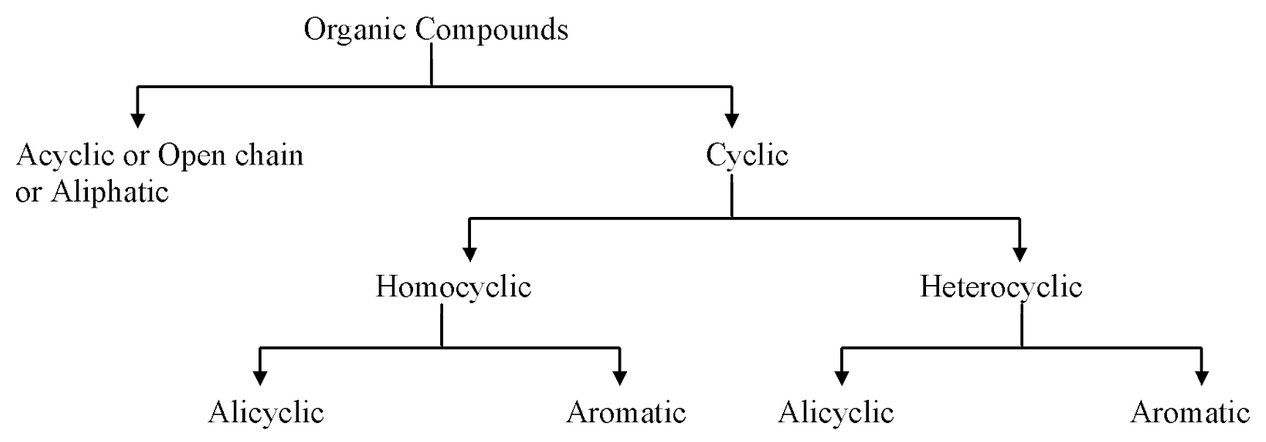
Aromatic Compounds
Aromatic compounds, often referred to as arenes, contain at least one planar, conjugated ring of pi electrons:
- Structure: Typically, a six-carbon ring with alternating single and double bonds.
- Example: Benzene (C6H6)
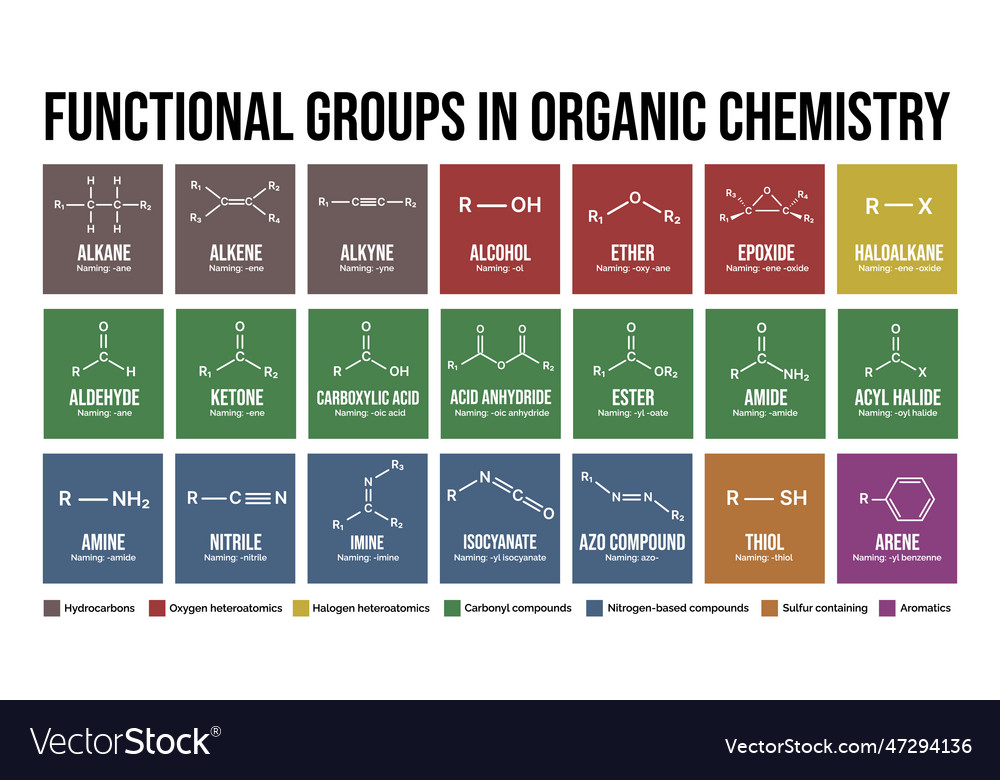
Functional Groups

Organic molecules often contain specific groups of atoms that influence their chemical properties, known as functional groups. Here are some common ones:
| Functional Group | Example | General Structure |
|---|---|---|
| Alcohols | Ethanol (C2H5OH) | -OH |
| Amines | Methylamine (CH3NH2) | -NH2 |
| Carboxylic Acids | Acetic Acid (CH3COOH) | -COOH |
| Esters | Ethyl Acetate (CH3COOCH2CH3) | -COOR |
| Ketones | Acetone (CH3COCH3) | C=O |

💡 Note: Functional groups play a critical role in determining the reactivity of organic compounds and their behavior in biological systems.
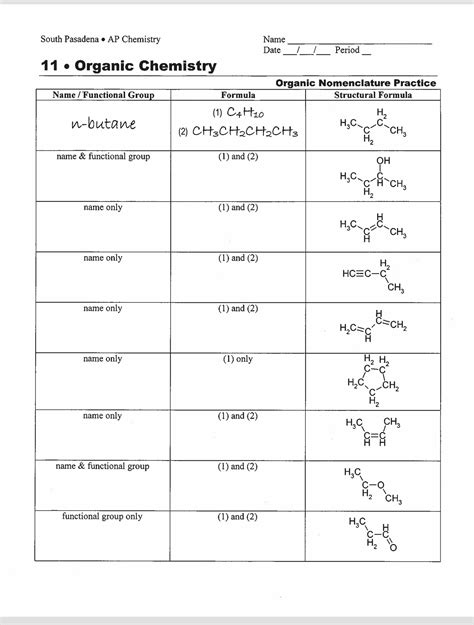
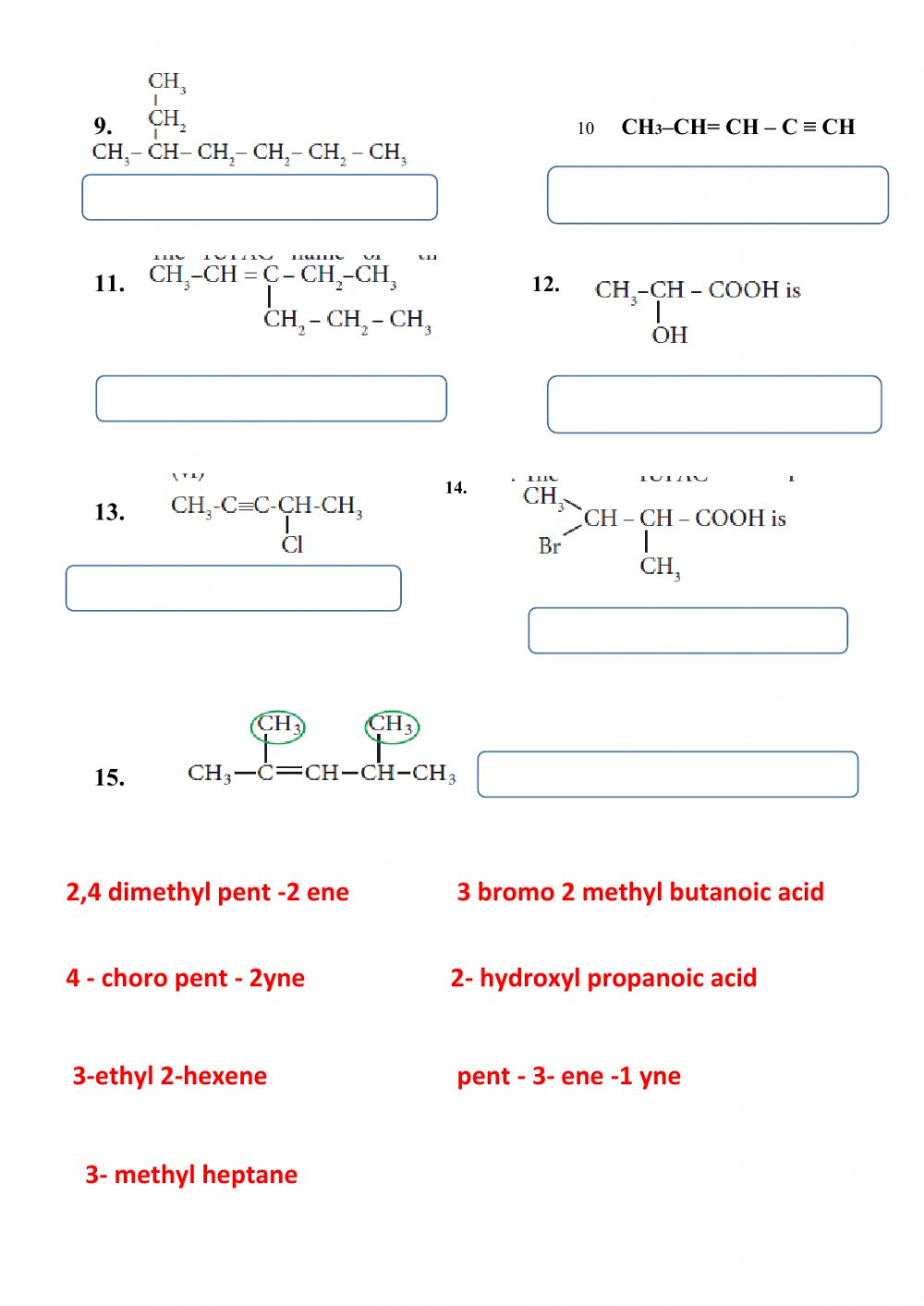
Nomenclature of Organic Compounds

Naming organic compounds is crucial for communication in chemistry. Here’s a brief guide:
- Identify the parent chain: the longest carbon chain containing the highest number of substituents.
- Number the chain to give the lowest possible numbers to the substituents.
- Name substituents with their prefixes (e.g., methyl-, ethyl-).
- Use the names for functional groups.
Applications and Examples
Here are some real-world applications and examples:
Pharmaceuticals

Many drugs are derived from or mimic organic compounds, like:
- Aspirin (acetylsalicylic acid), an ester and an alcohol.
- Antibiotics like Penicillin, which have a beta-lactam ring.
Polymers

Polymers are large molecules made up of repeating subunits:
- Polyethylene: Used in plastic bags and bottles.
- Polystyrene: Common in foam products and disposable cutlery.
Fuels
Alkanes serve as primary components in many fuels:
- Butane (C4H10) in lighters and portable stoves.
- Gasoline is primarily made from alkanes ranging from C5 to C12.
Organic compounds are fundamental to our daily lives, impacting industries, medicine, and the environment:
In closing, this exploration of organic compounds underscores their ubiquity and significance in chemistry and beyond. By mastering the basics of alkanes, alkenes, alkynes, aromatic compounds, and understanding the impact of functional groups, you’ve embarked on a journey that not only expands your knowledge but also connects you to the very chemistry of life itself. Keep exploring, learning, and appreciating the intricate world of organic chemistry.
What is the main difference between organic and inorganic compounds?

+
The primary difference is that organic compounds contain carbon bonded to hydrogen, while inorganic compounds usually do not, except in cases like carbon dioxide or carbonates.
How do functional groups affect the properties of an organic molecule?

+
Functional groups alter the reactivity, polarity, and other chemical properties of organic molecules by introducing different bonding patterns and electronic effects.
What is the importance of understanding the nomenclature of organic compounds?

+
Understanding nomenclature ensures clear and consistent communication within the scientific community about the structure and properties of specific compounds.

