Nuclear Decay Worksheet: Simplified and Engaging Guide for Students

The concept of nuclear decay or radioactivity is a fascinating part of atomic physics, where atomic nuclei undergo transformations, often releasing particles or energy in the process. This phenomenon can seem complex, but with the right tools and explanations, students can grasp it quite effectively. In this guide, we'll explore different types of nuclear decay, their effects, and practical ways to teach and understand these concepts through worksheets designed to engage and educate.
Nuclear Decay Basics
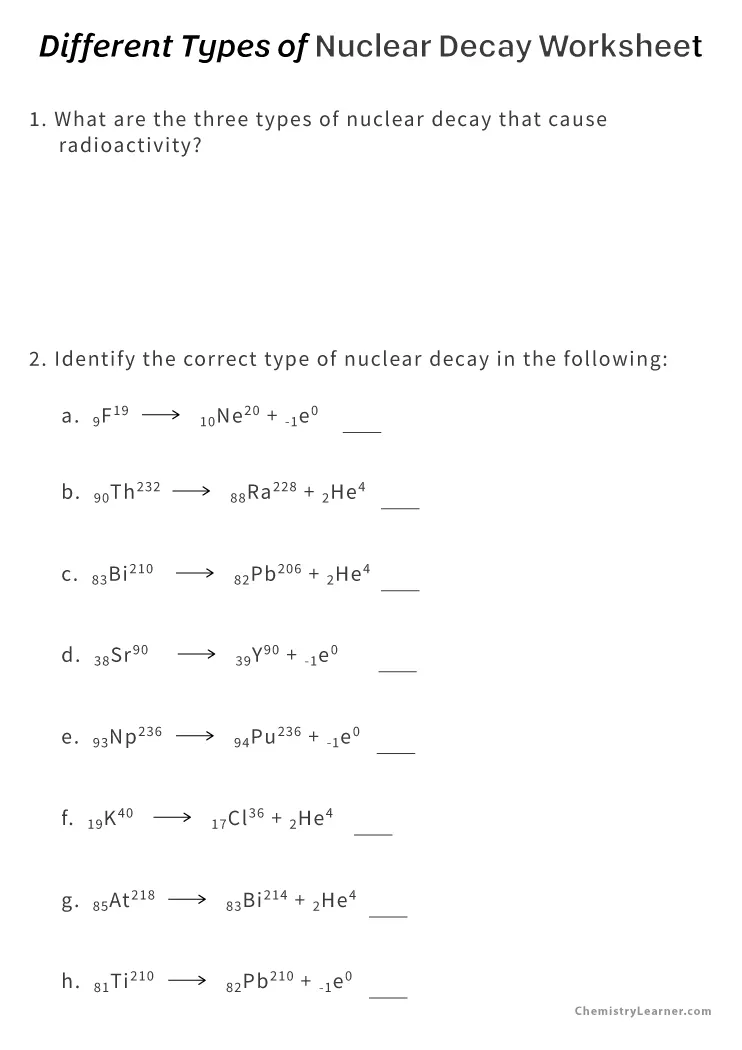
Atomic nuclei are composed of protons and neutrons. The balance between these particles can sometimes become unstable, leading to nuclear decay. Here are the primary types:
- Alpha Decay: Emission of an alpha particle (two protons and two neutrons) from the nucleus. This decreases the atomic number by two.
- Beta Decay: There are three types:
- Beta-minus (β−): A neutron is converted into a proton, an electron, and an antineutrino.
- Beta-plus (β+): A proton is converted into a neutron, a positron, and a neutrino.
- Electron capture: The nucleus absorbs an inner atomic electron, converting a proton into a neutron with the emission of a neutrino.
- Gamma Decay: This involves the emission of gamma rays (high-energy photons) to release excess energy from the nucleus, usually following another type of decay.
- Spontaneous Fission: Larger nuclei split into two or more lighter nuclei, sometimes releasing additional neutrons.
Developing an Engaging Worksheet
Creating a worksheet for nuclear decay needs to be both educational and interactive to capture students' interest. Here's how you can structure your worksheet:
1. Concept Introduction
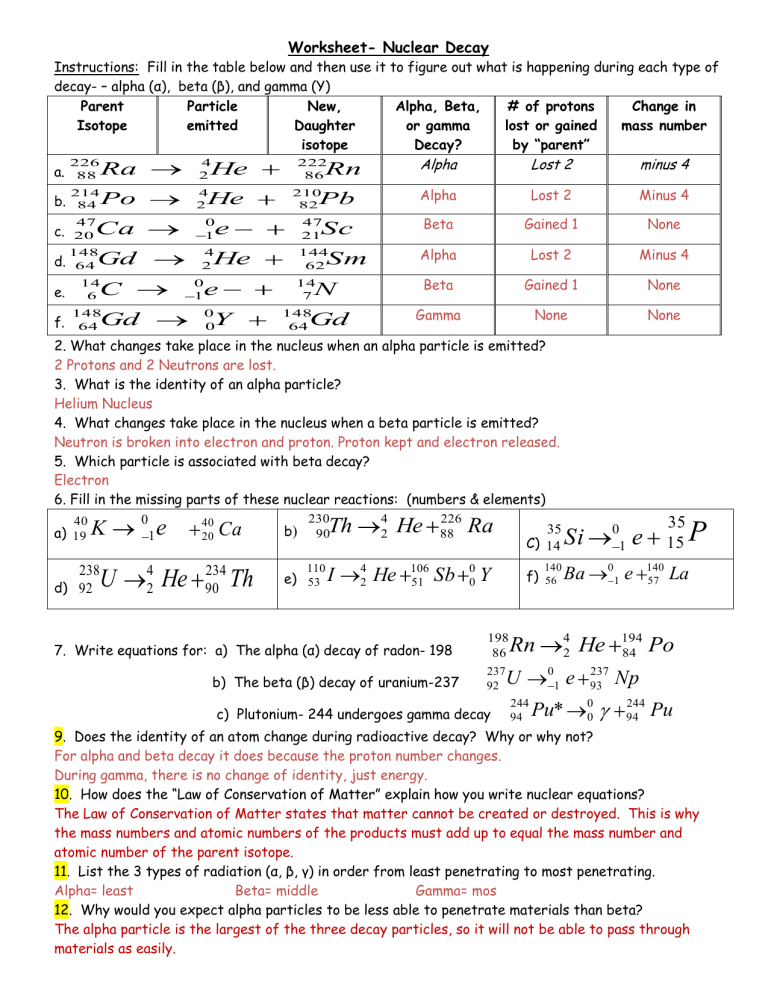
Start with a simple explanation:
Nuclear decay involves the changes in atomic nuclei to achieve stability. Here are some basic terms:
- Parent isotope: The unstable nucleus before decay.
- Daughter isotope: The product of the decay.
- Half-life: The time it takes for half of a sample of a radioactive substance to decay.
2. Activity Section

Include interactive elements:
- Word Search or Crossword Puzzles: Using terms related to nuclear decay.
- Fill in the Blanks: Provide students with sentences about decay types, requiring them to fill in the missing words or descriptions.
- Alpha, Beta, and Gamma Pathways: Diagram activities where students can trace the paths of alpha, beta, and gamma particles.
3. Problem-Solving Exercises

Offer quantitative problems:
Example: A certain element has a half-life of 5 years. If you start with 10 grams of this element, how much will remain after 15 years?
| Time (Years) | Amount Remaining (grams) |
|---|---|
| 0 | 10 |
| 5 | 5 |
| 10 | 2.5 |
| 15 | 1.25 |

⚠️ Note: Ensure that the problems are age-appropriate to challenge students without overwhelming them.
4. Experimental Design

Encourage students to think like scientists:
- Propose experiments where students can simulate radioactive decay using dice or coins to represent parent isotopes decaying into daughter isotopes over successive “half-lives.”
5. Real-World Application

Discuss practical applications:
- Carbon Dating: How decay helps to determine the age of organic materials.
- Medical Imaging: The use of radioactive isotopes for diagnostic purposes.
- Energy Production: Nuclear reactors and their reliance on nuclear decay processes.
🔬 Note: It’s important to connect the dots between the theory and real-world uses to illustrate the practical significance of nuclear decay.
In summary, understanding nuclear decay through an engaging and well-designed worksheet can significantly enhance students' comprehension of this complex subject. By integrating theory with hands-on activities, real-world applications, and problem-solving, educators can turn what might be perceived as a daunting topic into an exciting journey through atomic physics. Such methods not only foster a deeper understanding but also ignite curiosity and passion for science in young minds.
What are the primary types of nuclear decay?

+
The primary types are alpha decay, beta decay (including β−, β+, and electron capture), gamma decay, and spontaneous fission.
How does nuclear decay help in dating ancient objects?

+
Nuclear decay, specifically carbon dating, allows scientists to determine the age of organic materials by measuring the amount of remaining radioactive carbon-14 compared to stable carbon-12.
What are some safety measures when dealing with radioactive materials?

+
Safety measures include wearing protective gear, using shielded containers, minimizing exposure time, maintaining distance from sources, and adhering to protocols to avoid contamination.