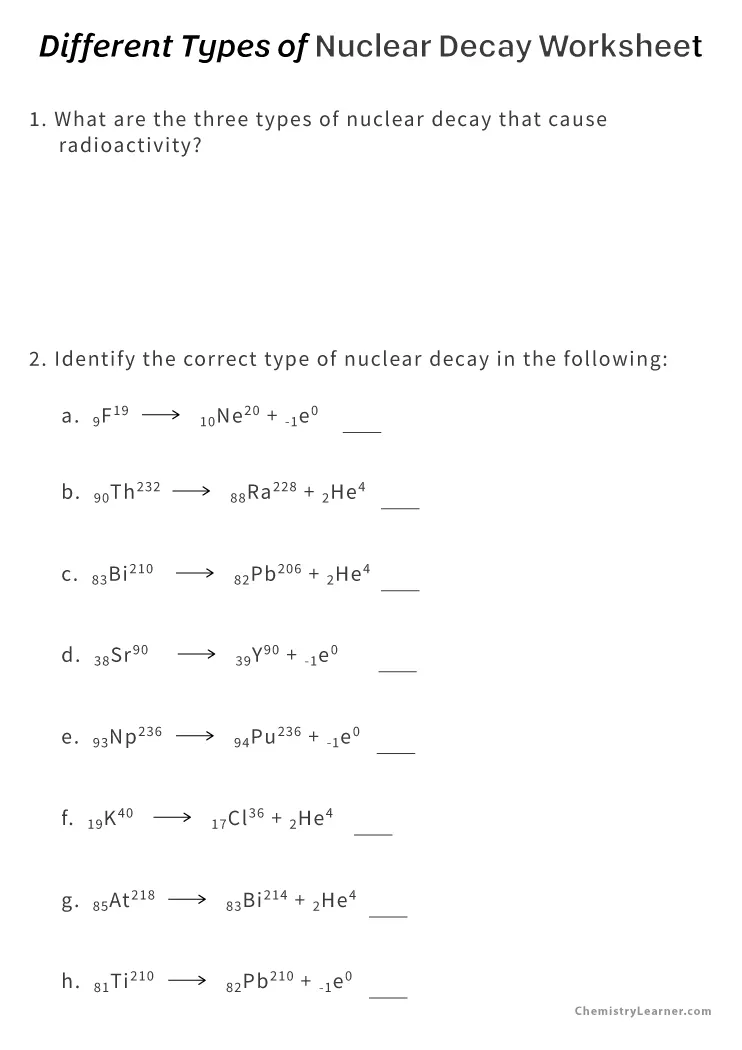
Master Nuclear Decay Reactions with Our Worksheet Answers

Understanding nuclear decay reactions is fundamental for anyone delving into the realm of nuclear physics or radiochemistry. Whether you're a student grappling with complex nuclear equations or a professional expanding your knowledge, mastering this topic can be both intriguing and challenging. This comprehensive guide will walk you through the process of solving nuclear decay reactions using our specially curated worksheet. Here, we'll not only cover the basics but also delve into advanced concepts, ensuring you grasp every aspect of nuclear decay.
What is Nuclear Decay?

Nuclear decay, also known as radioactive decay, is the process by which an unstable atomic nucleus loses energy by emitting radiation. This transformation can result in:
- Changes in the number of protons or neutrons in the nucleus.
- Emission of alpha particles, beta particles, or gamma rays.
The nucleus aims to reach a more stable state, and this process continues until it achieves stability or turns into a different element.
Types of Nuclear Decay

Before diving into the worksheet, it’s essential to understand the different types of nuclear decay:
- Alpha Decay (α): An alpha particle (which is essentially a helium-4 nucleus consisting of two protons and two neutrons) is emitted from the nucleus. The atomic number decreases by 2, and the mass number decreases by 4.
- Beta Minus Decay (β-): Here, a neutron turns into a proton, emitting an electron and an antineutrino. The atomic number increases by 1, but the mass number remains the same.
- Beta Plus Decay (β+): In this case, a proton transforms into a neutron, emitting a positron and a neutrino. The atomic number decreases by 1, and the mass number remains unchanged.
- Electron Capture: An inner shell electron is captured by the nucleus, resulting in a proton converting to a neutron with the emission of a neutrino.
- Gamma Decay (γ): Often accompanying other types of decay, gamma decay involves the emission of a high-energy photon, leaving the atomic and mass numbers unchanged but reducing the nucleus’s energy.
- Other types of decay include: Spontaneous fission, proton emission, neutron emission, and cluster decay, though they are less common in routine nuclear decay studies.
Nuclear Decay Equations

Each type of decay can be represented through specific equations:
| Type of Decay | Equation |
|---|---|
| Alpha Decay | AXZ → A-4YZ-2 + 4He2 |
| Beta Minus Decay | AXZ → AYZ+1 + e- + ν̅ |
| Beta Plus Decay | AXZ → AYZ-1 + e+ + ν |
| Electron Capture | AXZ + e- → AYZ-1 + ν |

Solving the Worksheet

Now that you’re familiar with the basics, let’s tackle our worksheet. Here, we’ll provide step-by-step solutions to various nuclear decay problems:
Problem 1: Alpha Decay

Consider the decay of Uranium-238 (U-238) to Thorium-234 (Th-234):
238U92 → 234Th90 + ?
Solution: An alpha particle, which is 4He2, is emitted. Thus:
238U92 → 234Th90 + 4He2
🔬 Note: Here, the atomic number decreases by 2, and the mass number decreases by 4.
Problem 2: Beta Minus Decay
A Carbon-14 (C-14) nucleus undergoes β⁻ decay:
14C6 → ? + e- + ν̅
Solution: The neutron within the C-14 nucleus turns into a proton, leading to:
14C6 → 14N7 + e- + ν̅
Problem 3: Electron Capture

Take the case of Potassium-40 (K-40) capturing an electron from its inner shell:
40K19 + e- → ?
Solution: After electron capture, a proton converts into a neutron:
40K19 + e- → 40Ar18 + ν
As you work through these examples, remember to balance mass and atomic numbers to keep the reaction equations accurate.
By now, you should have a better understanding of how to approach and solve nuclear decay reactions. Practice is key, so continue working on similar problems from our worksheet to reinforce your knowledge. If you're looking for more advanced concepts or if you face any challenges, consider exploring resources like textbooks, academic papers, or educational videos for further insights.
To recap:
- Nuclear decay involves various processes where unstable nuclei emit particles or rays to reach stability.
- Different types of decay result in different changes to the atomic structure.
- Balancing nuclear reactions requires understanding mass and atomic number conservation.
What are the primary types of nuclear decay?

+
The primary types of nuclear decay are alpha decay, beta minus decay, beta plus decay, electron capture, and gamma decay.
How do I balance a nuclear decay equation?

+
Balance a nuclear decay equation by ensuring that both mass numbers and atomic numbers on the left and right sides of the equation are equal. This includes accounting for particles emitted in the process.
Can you explain why some nuclei decay?

+
Unstable nuclei decay because they aim to reach a lower energy state, which is more stable. This occurs due to the balance between nuclear forces that hold the nucleus together and electrostatic repulsion between protons.


