5 Simple Tips to Master Nuclear Decay Equations

The study of nuclear reactions and radioactive decay can appear daunting initially, but with a focused approach, you can master the equations governing these processes. Here's how to understand and solve nuclear decay equations effortlessly.
Understand the Basics

Before diving into complex nuclear decay equations, familiarize yourself with the core principles:
- Atomic Number (Z): This represents the number of protons in an atom’s nucleus, influencing its chemical identity.
- Mass Number (A): This denotes the total number of protons and neutrons in the nucleus.
- Types of Decay: Be aware of alpha decay (α), beta-minus decay (β-), beta-plus decay (β+), and gamma emission (γ).
Learn the Decay Equations
Nuclear decay equations are mathematical expressions that describe how atoms change during radioactive decay. Here’s how different decay types alter an element:
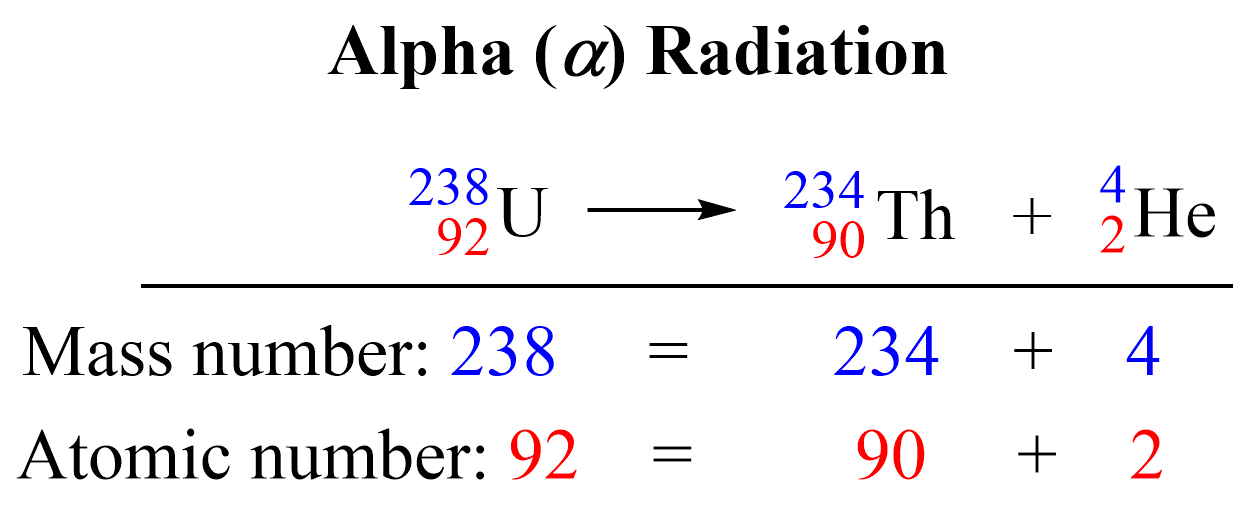
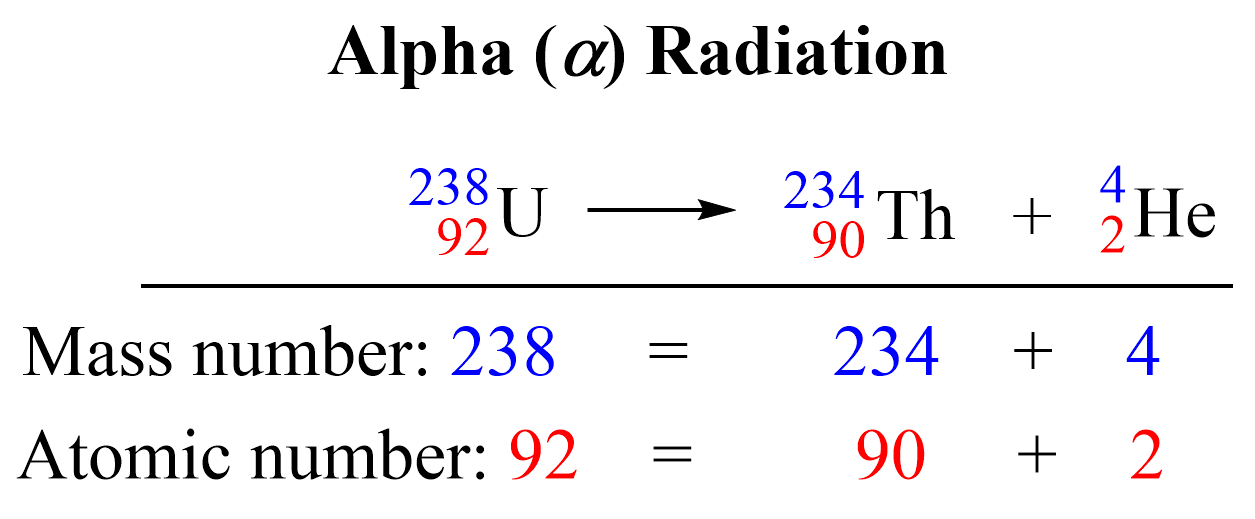
- Alpha Decay: Emits an alpha particle (2 protons and 2 neutrons), decreasing both atomic and mass numbers:
A → A-4, Z → Z-2 - Beta-Minus Decay: An electron is emitted, with a neutron transforming into a proton, increasing the atomic number by one:
A stays constant, Z → Z+1 - Beta-Plus Decay: Positron emission, where a proton becomes a neutron, reducing the atomic number:
A stays constant, Z → Z-1 - Gamma Emission: Involves releasing high-energy photons, leaving the atomic and mass numbers unchanged, but the nucleus transitions to a lower energy state.
⚛️ Note: Gamma emission often accompanies alpha or beta decay but does not change the element's identity.
Practice Writing Equations

Start by writing out balanced decay equations:
- Identify the original nuclide and the decay particle.
- Adjust the atomic and mass numbers as per the decay type.
- Use isotopic notation: ^A_X, where X is the element symbol, A is the mass number, and Z (atomic number) is a subscript.
Here’s an example:
²³⁸₉₂U → ²³⁴₉₀Th + ⁴₂α
Understand Conservation Laws

Nuclear decay must obey the laws of conservation:
- Charge Conservation: The total charge of the particles involved must remain unchanged.
- Mass-Energy Conservation: The sum of the masses and energies of particles before and after decay should be equal.
- Momentum Conservation: The total momentum of a system must be the same before and after a nuclear decay.
🔍 Note: When writing nuclear equations, mass-energy conservation can include loss due to the release of energy, which can be calculated using Einstein’s mass-energy equivalence.
Use Available Tools
There are numerous educational tools and applications that can assist in understanding and solving nuclear decay equations:
- Online Calculators: Many online tools can instantly balance nuclear equations for you.
- Educational Software: Programs like PhET Interactive Simulations can provide interactive visualizations of decay processes.
- Nuclear Decay Series Charts: Utilize charts showing decay chains of radioactive elements.
By mastering these five steps, you'll be well-equipped to understand and solve nuclear decay equations with confidence. Remember, consistency in practice is crucial. The more you engage with these concepts, the more intuitive they will become.
What is the difference between alpha, beta, and gamma decay?

+
Alpha decay involves the emission of an alpha particle, which decreases the atomic and mass numbers. Beta decay includes electron or positron emission, changing only the atomic number. Gamma decay releases high-energy photons, typically following alpha or beta decay, without altering the atomic or mass numbers.
Why does a nucleus undergo decay?
+
Nuclei decay to achieve a more stable configuration. This can mean reducing excess energy, balancing the proton-to-neutron ratio, or moving towards a configuration with lower energy states, which makes the nucleus more stable.
How can I predict the type of decay?

+
Predicting the exact type of decay can be challenging, but general rules exist. For example, if the nucleus has too many protons for stability, it’s likely to undergo beta-minus decay. Elements with very high atomic numbers might undergo alpha decay to reduce mass and charge.
What are decay chains?

+
Decay chains are series of decays where the product of one decay is the parent of the next. This continues until a stable isotope is reached. Uranium-238, for instance, has a long decay chain involving multiple alpha and beta decays before becoming stable lead-206.
Can we control nuclear decay?

+
Not directly; nuclear decay is a spontaneous process. However, through nuclear reactions, isotopes can be transmuted into others with potentially different decay properties, or the environment can be changed to influence the decay rate in certain cases (e.g., by temperature).