Chemical Formula Worksheet Answers: Simplify Your Study!
Understanding chemical formulas is a crucial part of mastering chemistry. Whether you're a high school student or an aspiring chemist, knowing how to interpret, balance, and name chemical compounds can streamline your study process and enhance your understanding of chemical reactions. This comprehensive guide is designed to offer detailed answers to commonly asked questions about chemical formulas, equipping you with the knowledge to excel in your chemistry studies.
Deciphering Chemical Formulas

Before diving into worksheets and answers, let’s first clarify what a chemical formula represents. A chemical formula:
- Identifies the type and number of atoms in a molecule or compound.
- Uses elemental symbols and subscripts to depict the composition.

Worksheet: Analyzing Chemical Formulas

Let’s analyze some typical questions from a chemical formula worksheet:
- What does the formula H2O tell us? It indicates that water molecules consist of two hydrogen atoms bonded to one oxygen atom.
- Calculate the molecular weight of C6H12O6 (glucose).
- Carbon © = 12.01 amu
- Hydrogen (H) = 1.008 amu
- Oxygen (O) = 15.999 amu
- Formula: (6 x 12.01) + (12 x 1.008) + (6 x 15.999) = 180.16 amu
- Write the formula for potassium sulfate. The formula is K2SO4.
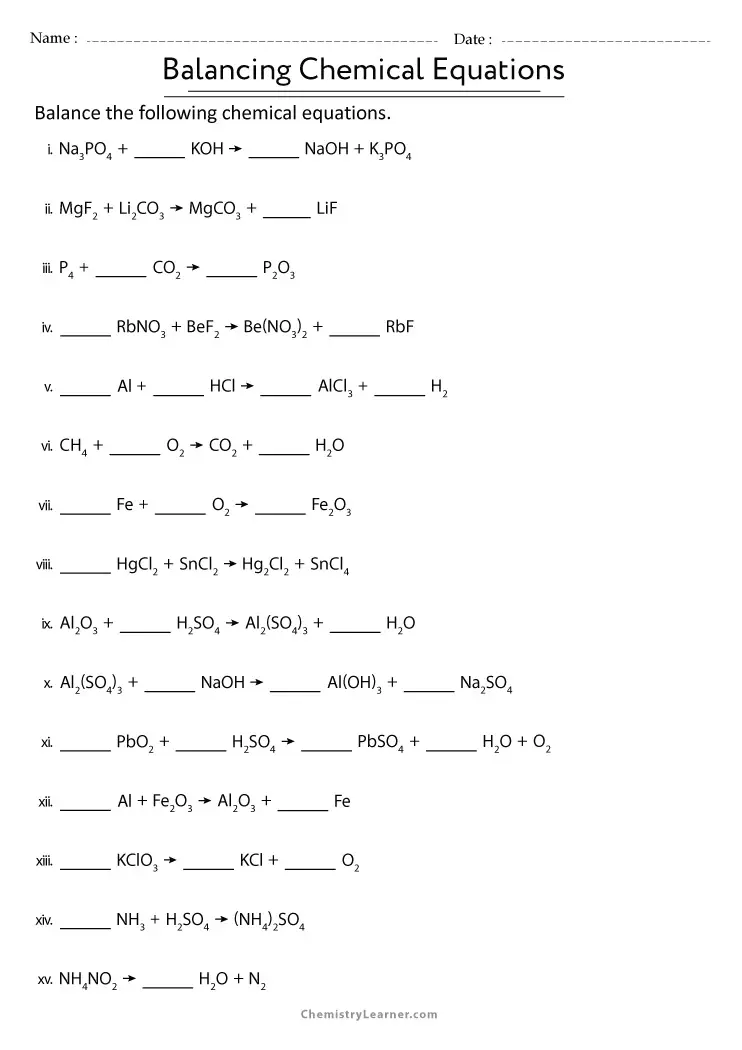
Balancing Chemical Equations

To balance chemical equations:
- Identify reactants and products: Determine the chemical formulas for all substances involved.
- Ensure atom conservation: Adjust the coefficients (not subscripts) to balance the number of atoms on both sides.
- Check for equality: Verify that each type of atom has the same count on both sides.
Here's an example:
| Reactants | Product | Balanced Equation |
|---|---|---|
| N2 + H2 | NH3 | N2 + 3H2 → 2NH3 |

Naming Chemical Compounds
Naming compounds requires knowledge of:
- Elemental names and symbols
- Prefixes for covalent compounds (e.g., mono-, di-, tri-)
- Roman numerals for transition metals to denote oxidation state (e.g., Cu(II), Cu(I))
For example, HCl is named as:
- Hydrogen chloride (when referring to the gas)
- Hydrochloric acid (when dissolved in water)
Recapitulation

In mastering chemical formulas, we've covered how to read and analyze them, balance equations, and appropriately name compounds. These skills are not only essential for understanding chemistry but also for solving complex chemical problems effectively. Applying this knowledge systematically to your studies will provide a solid foundation in chemistry, enabling you to tackle any worksheet or examination with confidence.
What’s the difference between empirical and molecular formulas?

+
An empirical formula shows the simplest whole-number ratio of atoms in a compound, whereas a molecular formula shows the actual number of atoms in a molecule.
How do you calculate the formula mass?

+
To find the formula mass, sum up the atomic masses of all the atoms in a chemical formula.
Why do we balance chemical equations?
+
We balance chemical equations to adhere to the law of conservation of mass, ensuring that the number of atoms of each element is the same on both sides of the equation.



