Nuclear Chemistry Review Worksheet Answers: Master the Concepts Easily

In the world of chemistry, understanding nuclear reactions and their applications is both challenging and fascinating. Whether you're a student preparing for an exam, or someone with a keen interest in nuclear science, mastering the concepts of nuclear chemistry can be made much easier with a bit of guidance. This detailed guide is tailored to provide clarity on various aspects of nuclear chemistry, offering answers to common worksheet questions and clarifying key concepts. Let's delve into the intricate details of nuclear chemistry to ensure you grasp the core principles effortlessly.
The Basics of Nuclear Chemistry

Before diving into complex reactions, it’s essential to understand the basics:
- Nuclear Reactions differ significantly from chemical reactions. While chemical reactions involve the rearrangement of electrons, nuclear reactions deal with changes within the nucleus.
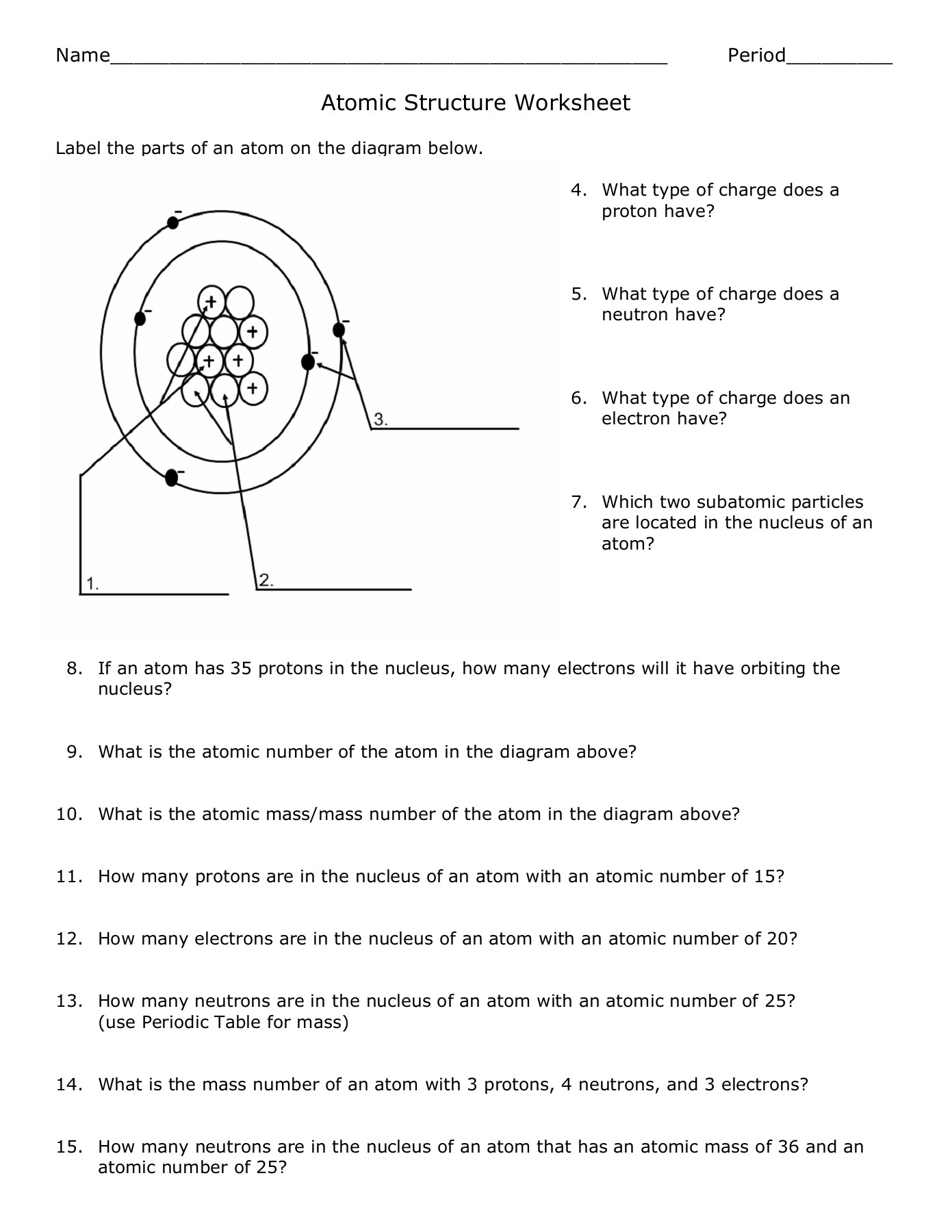
- Atomic Nucleus: The nucleus contains protons (positively charged) and neutrons (neutral). Their composition dictates the element’s identity and stability.
- Isotopes: These are variants of an element with the same number of protons but different numbers of neutrons.
🎯 Note: The stability of isotopes is determined by the neutron-to-proton ratio. Elements with higher atomic numbers tend to have a higher neutron-to-proton ratio for stability.
Nuclear Reactions

Understanding the types of nuclear reactions is fundamental:
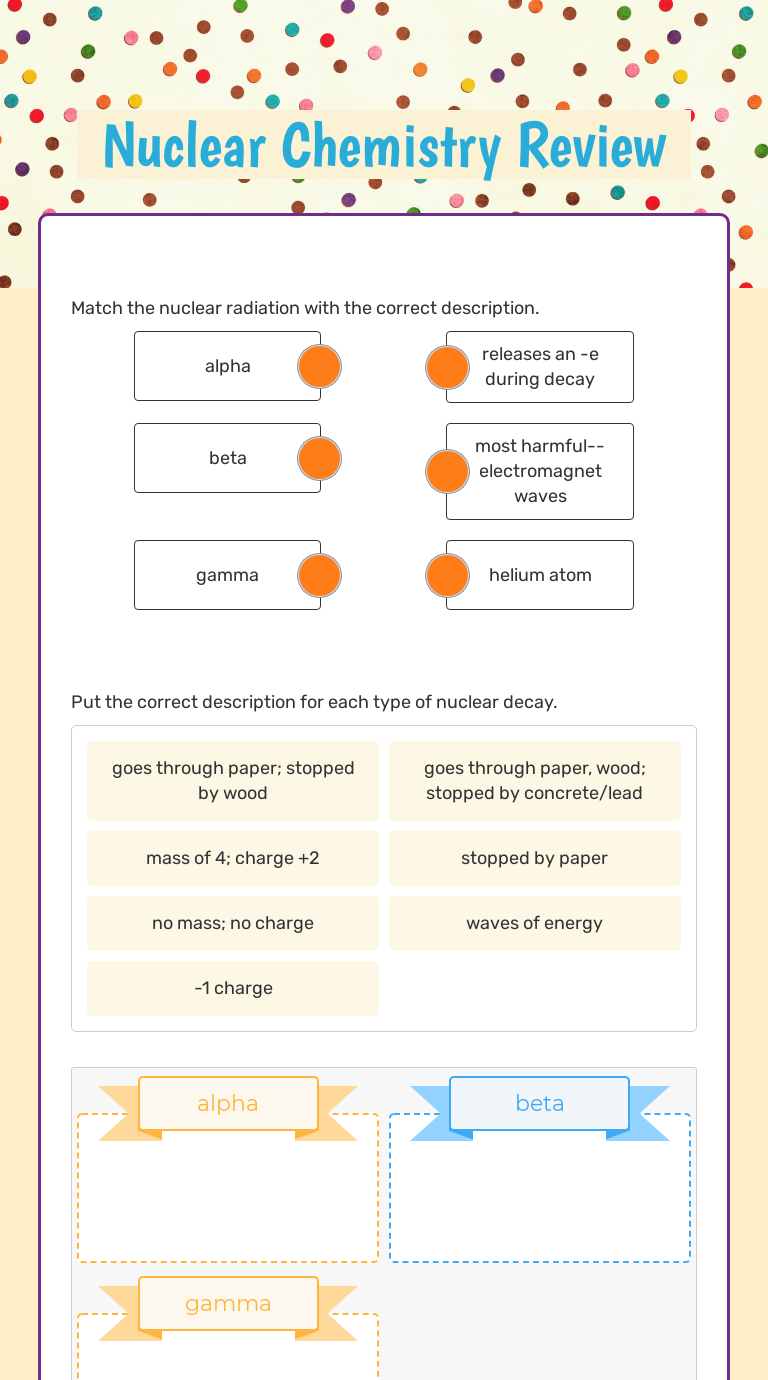
- Alpha Decay: When an unstable nucleus emits an alpha particle, which is equivalent to a helium nucleus (2 protons and 2 neutrons). The mass number decreases by 4, and the atomic number by 2.
- Beta Decay: Here, a neutron inside the nucleus converts into a proton, emitting an electron (β–) or a positron (β+). This changes the atomic number without altering the mass number significantly.
- Gamma Emission: This is pure energy emission without any change in the proton or neutron count.
| Nuclear Process | Change in Mass Number | Change in Atomic Number |
|---|---|---|
| Alpha Decay | -4 | -2 |
| Beta Minus Decay | 0 | +1 |
| Beta Plus Decay | 0 | -1 |
| Gamma Emission | 0 | 0 |

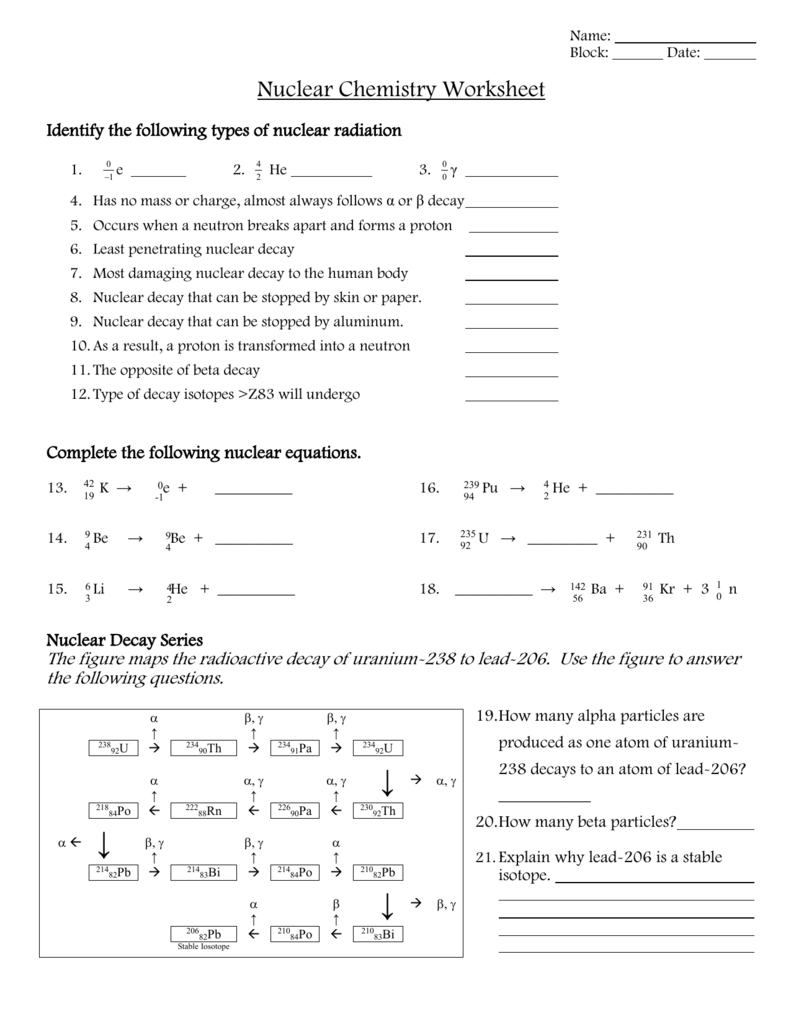
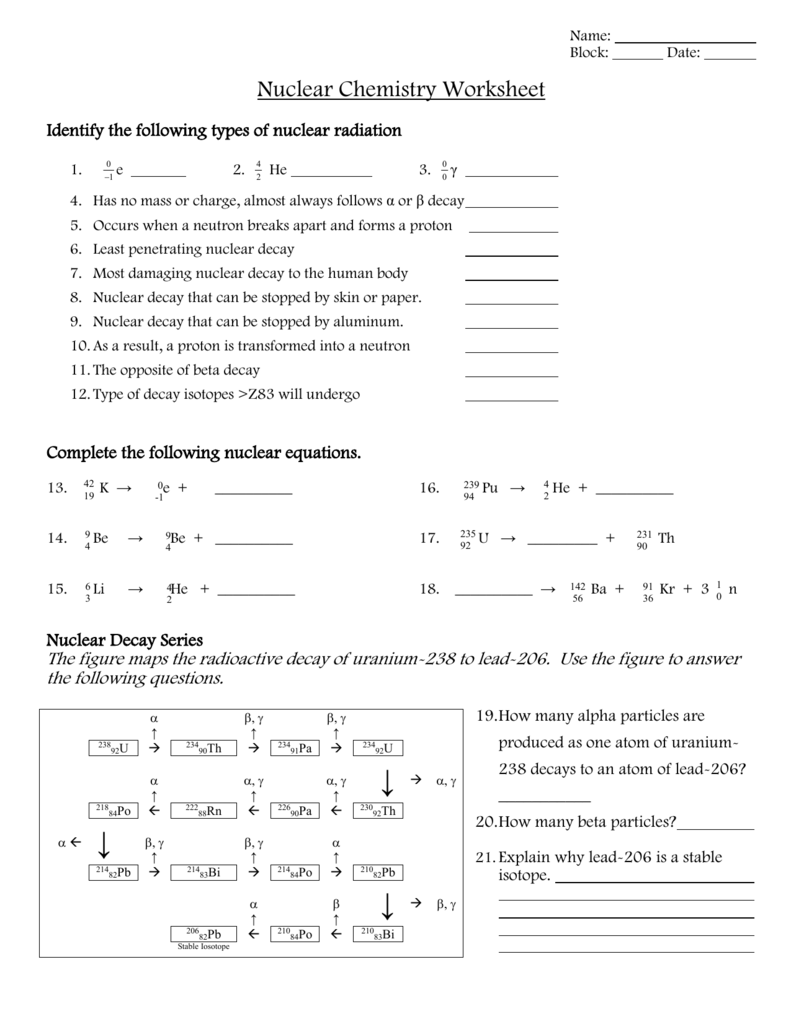
Radioactive Decay Series

Many radioactive elements undergo a series of decays, transforming into more stable isotopes through a process known as a decay series:
- Example: The uranium-238 decay series, which ultimately ends with lead-206 after numerous alpha and beta decays.
📌 Note: Decay chains often involve multiple steps due to the instability of intermediate isotopes.
Half-Life
The concept of half-life is pivotal:
- The half-life of a radioactive isotope is the time required for half of the radioactive atoms to decay. It remains constant for a specific isotope.
- Half-life measurements are critical for determining the age of geological formations or artifacts through radiometric dating.
Nuclear Fission and Fusion
These are processes that can release immense amounts of energy:
- Nuclear Fission: The splitting of a heavy nucleus into lighter ones, releasing energy and often more neutrons which can perpetuate the reaction.
- Nuclear Fusion: The process where light nuclei combine to form a heavier nucleus, with the release of energy. This is the process powering the stars, including our Sun.
Practical Applications

Nuclear chemistry finds its utility in various fields:
- Medical Diagnostics and Treatment: Radioisotopes are used in imaging techniques like PET scans, as well as in treating cancers through radiation therapy.
- Energy Production: Nuclear reactors harness fission for generating electricity, with fusion being researched as a potential future energy source.
- Archeology and Geology: Radiometric dating helps in dating ancient materials, understanding geological time scales, and analyzing past climates.
🌟 Note: The applications of nuclear chemistry have far-reaching impacts, influencing health, energy security, and our understanding of Earth’s history.
End of the Nucleus: A Recap
This review has provided a comprehensive look into nuclear chemistry, touching on the nature of nuclear reactions, decay processes, the concept of half-life, and the transformational power of fission and fusion. Understanding these processes not only broadens one’s knowledge of physical sciences but also opens doors to appreciating the technologies that have shaped modern civilization. Each step in nuclear chemistry, from decay series to energy production, presents unique challenges and opportunities, demonstrating the dynamic interplay between science and society. The continuous evolution in this field promises even more exciting developments in the future, making the study of nuclear chemistry both relevant and essential.
What is the difference between nuclear fusion and nuclear fission?

+
Nuclear fusion involves combining lighter atomic nuclei into heavier ones, releasing energy in the process. This is how stars generate their energy. Nuclear fission, in contrast, is the process of splitting a heavy nucleus into lighter nuclei, also releasing energy, and is used in nuclear reactors and atomic bombs.
How can nuclear chemistry be used in medicine?
+
Nuclear chemistry is used in medicine for diagnosing and treating diseases. For example, PET scans use short-lived positron-emitting isotopes to image body functions, and radiotherapy employs gamma rays or beta particles from radioactive substances to treat cancers.
What is meant by ‘half-life’ in nuclear chemistry?

+
The ‘half-life’ of a radioactive isotope is the time it takes for half of the atoms of the isotope to undergo radioactive decay. This term is crucial for calculating the age of organic and geological materials using radiometric dating techniques.