Atom Structure Worksheet: Key Answers Revealed

Understanding the structure of an atom is fundamental for students delving into the world of chemistry. This comprehensive worksheet not only explores the essential components of an atom but also provides detailed explanations to ensure a solid grasp of atomic theory. Here, we'll reveal the key answers for an Atom Structure Worksheet, offering insight into atomic number, atomic mass, isotopes, and electron configuration.
Atomic Number and Atomic Mass


Let's start by demystifying the concept of atomic number and atomic mass:
- Atomic Number: This is the number of protons in the nucleus of an atom. Each element has a unique atomic number, which defines its place in the periodic table.
- Atomic Mass: This value represents the sum of protons and neutrons in the nucleus. It's often close to but not exactly whole numbers due to the existence of isotopes.
To find these:
- Identify the atomic number (Z) directly from the periodic table or given in the problem. For example, Oxygen has an atomic number of 8.
- Calculate atomic mass by adding the number of protons and neutrons. For instance, an isotope of Carbon-14 has 6 protons and 8 neutrons; thus, its atomic mass is 14.
🔍 Note: When calculating atomic mass, ensure you're considering the correct isotope!
Isotopes
Isotopes are atoms of the same element with different numbers of neutrons. Here's how to determine the number of protons, electrons, and neutrons in isotopes:
- Isotope Notation: The notation for an isotope shows the mass number (total number of protons and neutrons) as a superscript and the atomic number as a subscript. For instance, 14C6 for Carbon-14.
- Calculations:
- Protons: Equal to the atomic number (6 for Carbon).
- Electrons: Equal to the number of protons unless the atom is charged (ion).
- Neutrons: Mass number minus atomic number (8 for Carbon-14).
🔬 Note: Isotopes have the same chemical properties because their electron configuration remains unchanged.
Electron Configuration
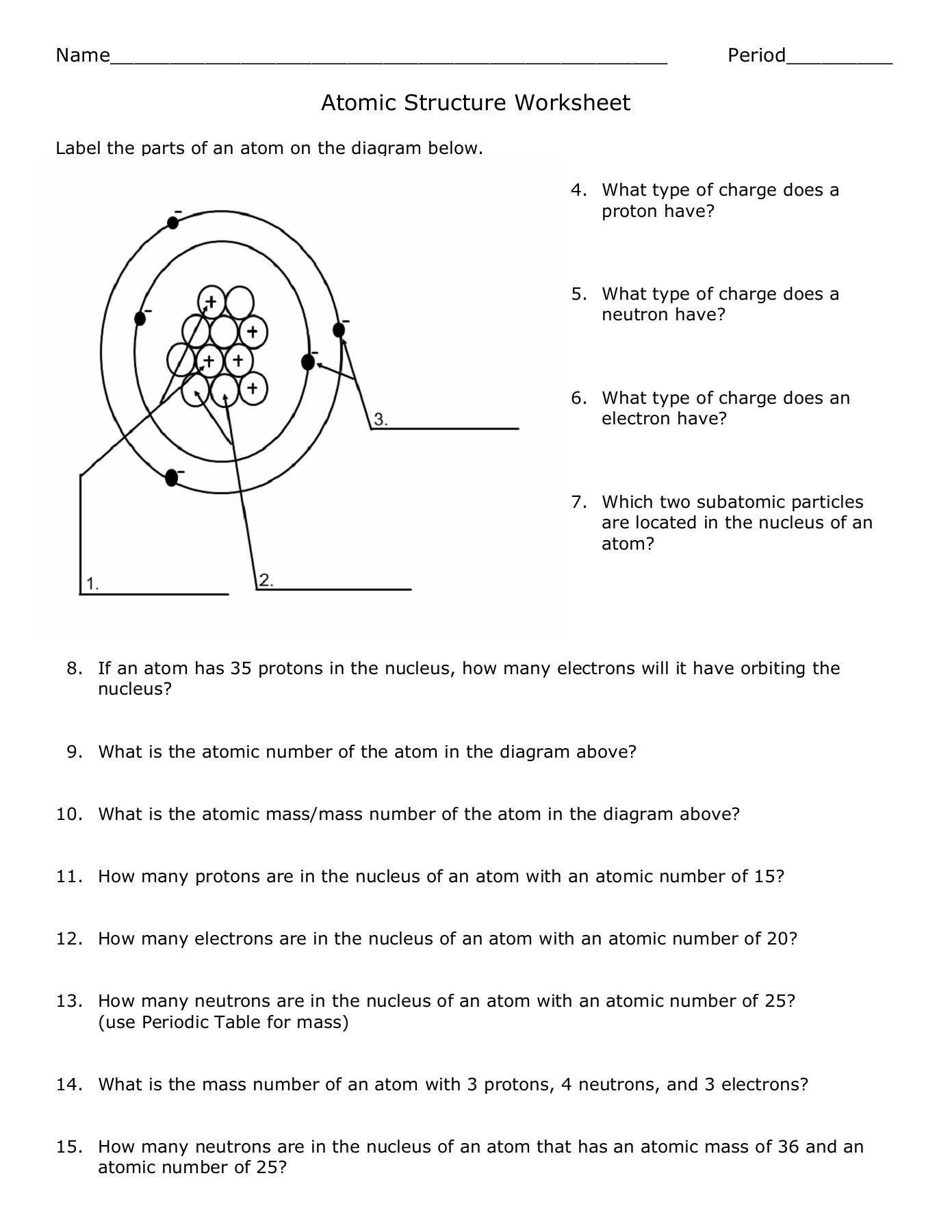
Electron configuration describes how electrons are arranged around the nucleus in different energy levels. Here’s how to represent it:
- Each element has a unique electron configuration based on the Aufbau principle, Pauli exclusion principle, and Hund's rule.
- The noble gas notation simplifies the electron configuration by using the last noble gas in the configuration to represent the inner electrons.
| Element | Atomic Number | Electron Configuration | Noble Gas Notation |
|---|---|---|---|
| Oxygen (O) | 8 | 1s22s22p4 | [He]2s22p4 |
| Calcium (Ca) | 20 | 1s22s22p63s23p64s2 | [Ar]4s2 |

Here's a step-by-step guide to writing electron configuration:
- Find the element's atomic number and follow the Aufbau principle to fill electron orbitals.
- Follow Hund's rule to distribute electrons in degenerate orbitals.
- Ensure no more than two electrons occupy an orbital (Pauli exclusion principle).
📝 Note: Remember the aufbau sequence: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s...
In summary, this Atom Structure Worksheet offers a deep dive into understanding atoms' basic structure, isotopes, and electron distribution. We've covered how to identify atomic numbers and atomic mass, how isotopes affect these values, and how to depict electron configurations effectively. Mastering these concepts equips students with essential tools for advanced chemistry topics and real-world applications in diverse fields.
What is the difference between an atom and an isotope?

+
An atom is the basic unit of matter, characterized by its atomic number, which indicates the number of protons. An isotope is a variant of an atom with a different number of neutrons, thus having a different atomic mass but the same atomic number.
Why is electron configuration important?

+
Electron configuration determines an atom’s chemical behavior, including reactivity, the bonds it can form, and its place in the periodic table. Understanding electron configuration is crucial for predicting how an element will interact chemically with others.
How can you calculate the number of neutrons from an isotope notation?

+
The number of neutrons can be calculated by subtracting the atomic number (the number of protons) from the mass number given in the isotope’s notation.