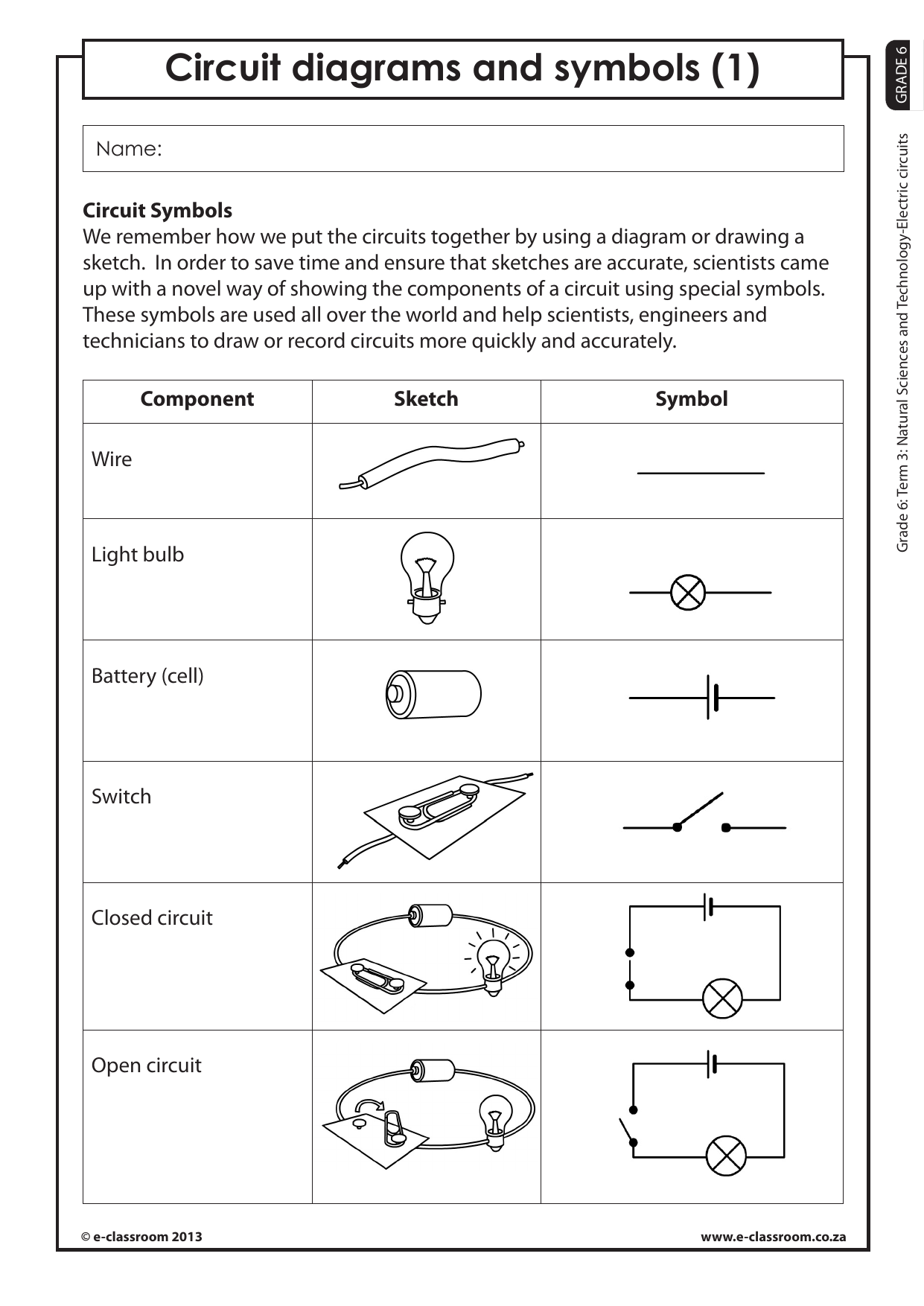
5 Ways to Solve Nova Poisoned Water Worksheet

In the world of chemistry and environmental science, understanding how to solve problems related to water contamination is crucial. The "Nova Poisoned Water" worksheet offers students and professionals a chance to apply theoretical knowledge to real-life scenarios. Here's how you can approach solving these intriguing challenges using five distinct methods:
Method 1: Chemical Neutralization

The first strategy to detoxify contaminated water is through chemical neutralization. This method involves:
- Identifying the Contaminant: Determine what substances are present in the water. Common contaminants include heavy metals, acids, or bases.
- Choosing the Right Neutralizer: For acidic water, you might use bases like sodium bicarbonate or lime. For basic water, use acids like hydrochloric acid or sulfuric acid.
- Applying the Neutralizer: Add the neutralizing agent gradually while monitoring the water’s pH to avoid over-correction.
- Testing: After neutralization, test the water for contaminants and pH balance to ensure it is safe.
Example:

If you identify copper ions as the pollutant, adding a base like sodium hydroxide might precipitate copper out as copper(II) hydroxide:
🔬 Note: Always ensure to dilute acids or bases properly before addition to prevent rapid reactions that could lead to equipment damage or safety hazards.
Method 2: Adsorption

Adsorption is a process where pollutants are removed from water by adhering them to a solid surface. Here’s how to go about it:
- Selecting an Adsorbent: Common materials include activated carbon, zeolite, or clays which have a high surface area.
- Pre-treatment: Sometimes, water might require pre-treatment like sedimentation or filtration to remove larger particles.
- Adsorption Process: Pass the water through an adsorption column or stir it with the adsorbent in a container.
- Regeneration and Disposal: Once the adsorbent is saturated, it can either be regenerated or disposed of properly.
The effectiveness of this method lies in the adsorption capacity of the material used:
| Adsorbent | Capacity for Organic Compounds (mg/g) | Capacity for Heavy Metals (mg/g) |
|---|---|---|
| Activated Carbon | 100 - 1000 | 0.1 - 50 |
| Zeolite | Not Significant | 50 - 150 |

Method 3: Biological Treatment

Biological treatment utilizes microorganisms to degrade or metabolize contaminants. Here’s how to implement this method:
- Understanding Microbes: Choose appropriate microorganisms based on the type of pollutant. Bacteria like Pseudomonas putida are effective in breaking down organic compounds.
- Providing Optimal Conditions: Ensure the conditions (pH, temperature, oxygen levels) are suitable for the microbes to thrive.
- Creating a Biofilter: Construct a system where water can be treated by passing through a bed of microbes.
- Monitoring: Regularly check water quality and microbial activity to maintain treatment efficiency.
Method 4: Membrane Filtration

This involves using membranes to filter out contaminants based on their size:
- Membrane Selection: Different types of membranes like reverse osmosis (RO), nanofiltration (NF), or microfiltration (MF) can be chosen based on the pollutant's molecular weight.
- Pressure Management: Apply appropriate pressure to force water through the membrane, separating clean water from pollutants.
- Maintenance: Regular cleaning or replacement of membranes is essential to ensure filtration efficiency.
Membrane filtration can also be visualized with:
| Membrane Type | Pore Size (nm) | Applications |
|---|---|---|
| Microfiltration (MF) | >100 | Bacteria, yeast, other large particles |
| Nanofiltration (NF) | 1-10 | Organic molecules, small bacteria |
| Reverse Osmosis (RO) | <1 | Ions, small molecules, viruses |
Method 5: Ion Exchange

Ion exchange involves replacing harmful ions in water with benign ones:
- Resin Selection: Use resins that can exchange ions of concern. An example is using a cationic resin to remove heavy metals.
- Column Setup: Water is passed through columns packed with ion exchange resin, where the exchange occurs.
- Regeneration: The resin can often be regenerated by flushing with a strong acid or base, depending on the ion originally exchanged.
- Disposal: Proper disposal of the regeneration solution is critical to prevent further pollution.
In summary, each of these methods for solving the Nova Poisoned Water Worksheet presents unique benefits and challenges. Chemical neutralization is straightforward for adjusting pH levels, while adsorption excels in removing organic compounds and heavy metals. Biological treatments utilize nature's own cleanup crew, membrane filtration offers precision based on size, and ion exchange allows for targeted removal of specific ions. By understanding the nature of the contaminant, you can select and adapt these methods to create a tailored water treatment solution, ensuring both the safety of the water and the environment.
What should I consider when choosing a water treatment method?

+
Consider the type of pollutant, the water volume, the feasibility of the method in terms of cost and resources, environmental impact, and the end-use of the treated water.
Is any single method the best for all types of water contamination?

+
No, each method has its strengths and weaknesses. Often, a combination of methods might be the most effective for comprehensive treatment.
How can I ensure that my treated water is safe for consumption?

+
After treatment, the water should undergo rigorous testing for contaminants like bacteria, heavy metals, pesticides, etc., to ensure it meets drinking water standards.