Master Binary Covalent Compounds Nomenclature Easily

Delving into the world of chemistry, understanding the nomenclature of binary covalent compounds is a fundamental skill. These compounds, composed of two different elements bonded covalently, require a systematic approach for naming, ensuring clarity and consistency in communication. In this comprehensive guide, we'll unravel the nomenclature rules, offer practical examples, and provide tips to master this essential topic.
Understanding Binary Covalent Compounds
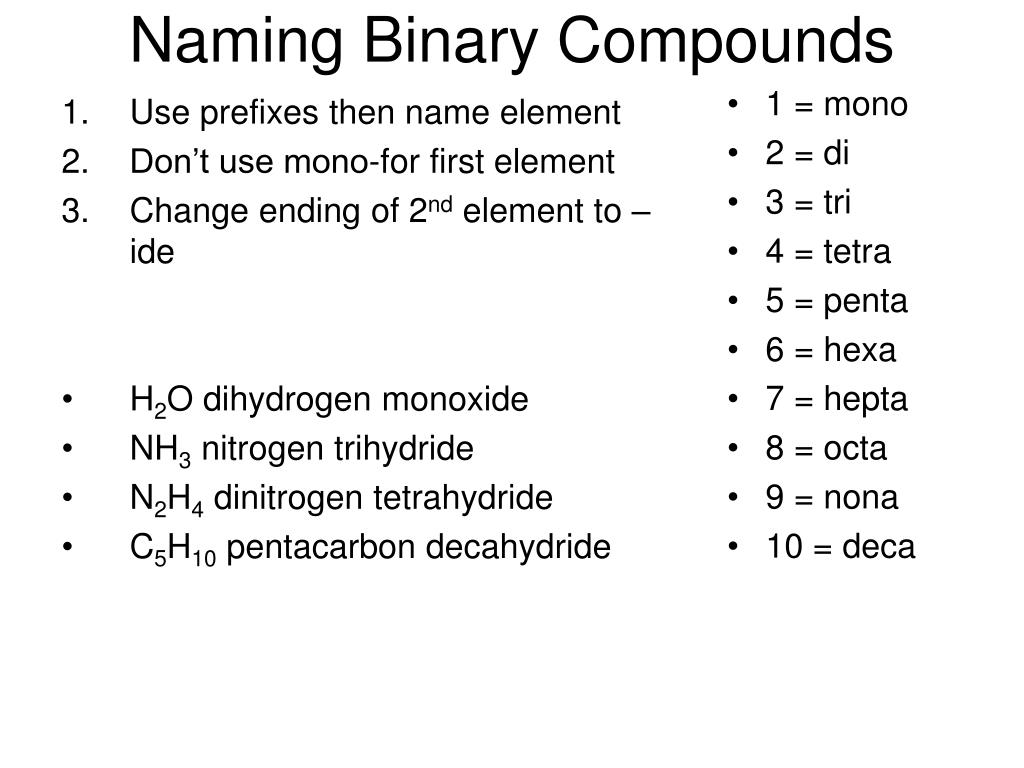
Binary covalent compounds consist of two nonmetals sharing electrons. The challenge lies in identifying and naming these compounds accurately, as their composition doesn't follow the same pattern as ionic compounds. Here are key points to understand:
- Covalent Bonding: These compounds involve the sharing of electrons, not the transfer, making them different from ionic compounds.
- Nonmetal Elements: Only nonmetals form binary covalent compounds. Recognizing which elements are nonmetals is crucial.
- Molecular Formulas: The formula of these compounds represents the actual number of atoms of each element in a molecule.

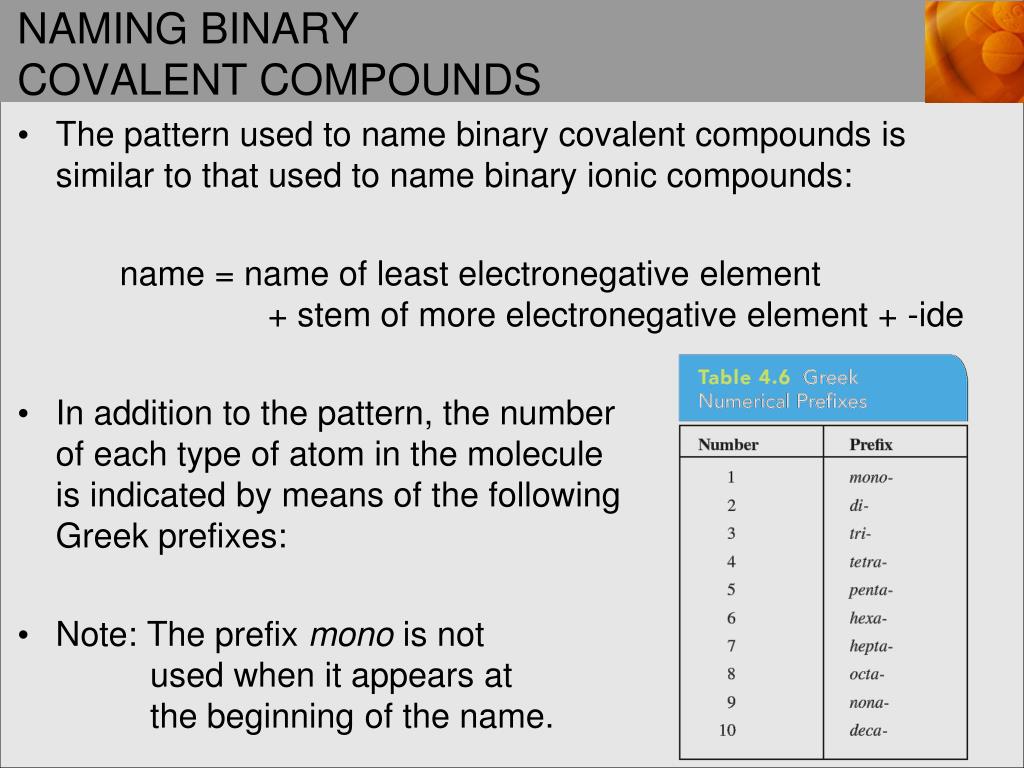
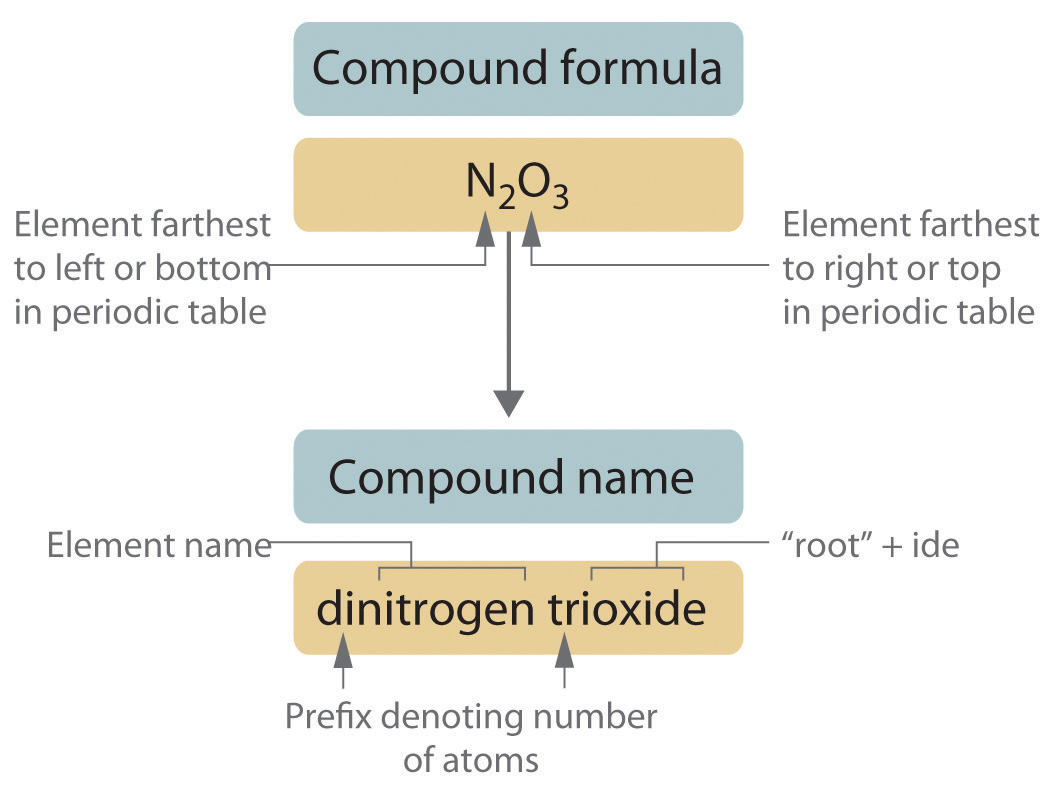
Naming Rules for Binary Covalent Compounds

Naming these compounds involves following specific rules:
- The First Element: The first element in the formula is named directly, without any numerical prefixes unless it's a diatomic molecule like Cl₂ or O₂, which are named as chlorine or oxygen, respectively.
- The Second Element:
- Is named with an “-ide” suffix.
- Receives numerical prefixes to indicate the number of atoms present, with a few exceptions (like H₂O).
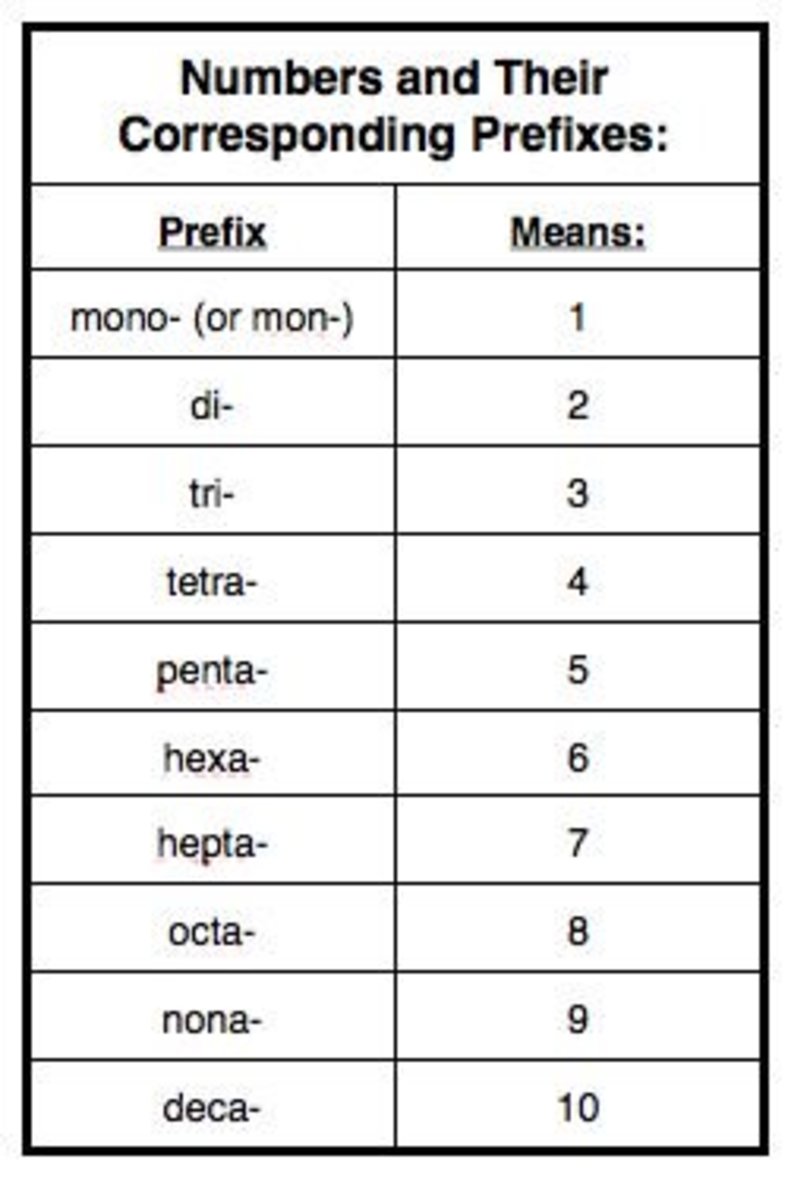
- Numerical Prefixes: Use Greek numerical prefixes to indicate the number of atoms of each element. Here's the list:
Number Prefix 1 Mono 2 Di 3 Tri 4 Tetra 5 Penta 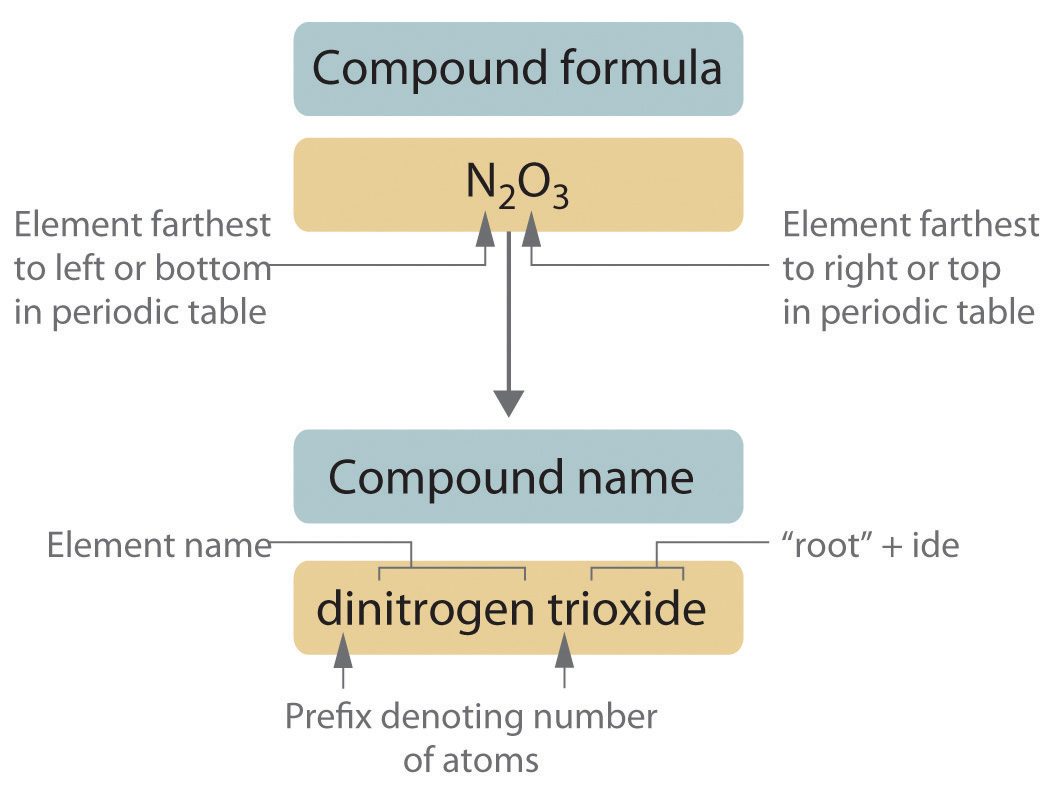
- Omission of Mono: “Mono” is usually omitted for the first element, but not for the second (e.g., CO is carbon monoxide).
- Parenthesis and Hyphens: Use parentheses for polyatomic ions in binary ionic compounds, but not for binary covalent compounds; use hyphens to connect prefixes to element names.
Examples and Practice
Let's delve into some examples to solidify our understanding:
- CO₂: Carbon dioxide (dioxide indicates two oxygen atoms).
- N₂O: Dinitrogen monoxide (dinitrogen indicates two nitrogen atoms).
- PCl₅: Phosphorus pentachloride (pentachloride indicates five chlorine atoms).
- H₂O: Water. Note that this compound breaks the rule by not using prefixes.
Tricky Compounds
Some compounds can be tricky due to:
- Ambiguity in Formula Writing: The same chemical formula can represent different compounds due to isomerism.
- Ambiguity in Naming: The use of common names like water instead of systematic names.
- Diatomic Elements: Elements like nitrogen (N₂) or hydrogen (H₂) which are diatomic in nature but not compounds.

Mastering the Nomenclature
To become proficient in naming binary covalent compounds:
- Practice Regularly: Consistent practice with a variety of compounds helps in recognizing patterns and exceptions.
- Use Mnemonics: Create mnemonics to remember common prefixes or exceptions.
- Understand Common Exceptions: Compounds like water, hydrogen peroxide, and ammonia have their own special names.
- Understand Bonding: A good grasp of how elements bond covalently will aid in naming and predicting properties.
💡 Note: While learning the names of compounds, don't forget to consider their context, like their use in industrial or environmental applications, which can help in memorization.
Common Mistakes and How to Avoid Them

Here are some common pitfalls to watch out for:
- Misuse of Prefixes: Not applying the correct prefix for the second element or forgetting to use them altogether.
- Confusion with Ionic Compounds: Ionic compounds have different naming rules, often leading to confusion with covalent compounds.
- Naming Elements Incorrectly: Misnaming elements like sulfur as sulf, or phosphorous as phosphor.
By understanding these mistakes and practicing, you'll increase your accuracy in naming binary covalent compounds.
In summary, mastering binary covalent compounds nomenclature involves understanding the basic rules, recognizing exceptions, and practicing. With a systematic approach, you can confidently name these compounds, which is crucial not only for academic success but also for understanding chemical reactions, industrial processes, and environmental chemistry. Remember that while these rules are general, always keep in mind the unique properties of certain compounds like water or ammonia, which provide valuable lessons in chemical nomenclature and bonding.
What makes a compound covalent?
+
A compound is covalent when it is formed by the sharing of electrons between two nonmetal atoms. This contrasts with ionic compounds, where electrons are transferred between a metal and a nonmetal.
How can I differentiate between binary ionic and binary covalent compounds?

+
Binary ionic compounds typically involve a metal and a nonmetal, where the metal loses electrons to the nonmetal. Binary covalent compounds, on the other hand, involve two nonmetals that share electrons. The key difference is in the type of bonding and the nature of elements involved.
Why is it important to know the number of atoms in a molecule?

+
Knowing the number of atoms helps predict the physical and chemical properties of a compound, how it reacts, its molecular structure, and its stoichiometry in chemical equations.



