5 Easy Steps to Master Simple Binary Ionic Compound Nomenclature

Understanding the nomenclature of simple binary ionic compounds is essential for anyone delving into the world of chemistry. These compounds, composed of a metal and a non-metal, form through ionic bonds where electrons are transferred from the metal to the non-metal. Mastering their naming conventions can seem daunting at first, but by following a structured approach, you can easily become proficient. Here’s a comprehensive guide to mastering simple binary ionic compound nomenclature in five easy steps.
1. Identify the Cations and Anions

The first step in naming a binary ionic compound is to identify the components of the compound - the metal cation (positive ion) and the non-metal anion (negative ion). This differentiation is crucial because the name of the compound will reflect the identity of these ions.
- Cations: Usually from metals, which lose electrons to become positively charged. Elements like sodium (Na+), potassium (K+), and magnesium (Mg2+) are common examples.
- Anions: Non-metals, which gain electrons to become negatively charged. Chlorine (Cl-), oxygen (O2-), and sulfur (S2-) are typical anions in binary compounds.
2. Name the Cation

The cation’s name remains largely unchanged from the element’s name unless it has variable charges:
- If the cation is from a metal with only one possible charge (like sodium, Na+, or calcium, Ca2+), use the element name directly.
- When dealing with transition metals or metals with variable oxidation states, use a Roman numeral following the name to indicate the charge. For example, iron could be Fe2+ (iron(II)) or Fe3+ (iron(III)).
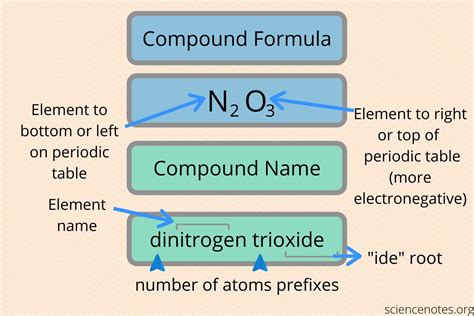
3. Name the Anion

Anions are named by changing the suffix of the element’s name to -ide:
- For example, Chlorine becomes chloride (Cl-).
- Some common anions include oxide (O2-), nitride (N3-), and sulfide (S2-).
⚗️ Note: When dealing with polyatomic ions, the suffix -ite or -ate is used to indicate fewer or more oxygen atoms respectively, but for simple binary compounds, the -ide suffix rule applies universally.
4. Combine the Names

Once you’ve identified and named the cation and anion, combine them in the following format:
- [Cation Name] + [Anion Name with -ide Suffix]
- For example, Sodium chloride (NaCl), Magnesium oxide (MgO), or Iron(III) sulfide (Fe2S3).
| Cation | Anion | Compound |
|---|---|---|
| Sodium | Chlorine | Sodium chloride |
| Calcium | Fluorine | Calcium fluoride |
| Copper(II) | Nitrogen | Copper(II) nitride |

5. Practice

The key to mastering the nomenclature of binary ionic compounds is practice. Here are some strategies to reinforce your understanding:
- Practice Naming: Write out the names for different combinations of common cations and anions.
- Memorize Charges: Spend time learning the common charges of elements, especially metals with multiple charges.
- Flashcards: Use flashcards to test your recall of ions and their corresponding names.
- Online Resources: Engage with interactive tools and quizzes available on chemistry websites.
By following these steps, you can effectively learn and apply the principles of naming binary ionic compounds. Not only will this enhance your understanding of chemical bonding, but it will also improve your proficiency in reading and interpreting chemical formulas in various applications, from pharmaceuticals to environmental science.
What is the importance of naming compounds correctly?

+
Correct naming is crucial in chemistry for clear communication among scientists, ensuring consistency in research, manufacturing, and education. It helps in understanding the composition, reactivity, and properties of substances.
Why do some metals need Roman numerals in their names?

+
Metals like iron, copper, and tin can exist in multiple oxidation states, and Roman numerals are used to indicate which state the metal is in for the compound in question, clarifying its charge.
Can a binary ionic compound have more than one anion?
+
Yes, if the cation has a higher charge than the anion, the compound can have more than one anion to balance the charges, e.g., in aluminum oxide (Al2O3), each aluminum atom has a charge of +3, requiring three oxygen atoms to balance the -2 charge of each oxide ion.



