Net Ionic Equations Simplified: Worksheet with Answers

In the fascinating world of chemical reactions, net ionic equations simplify the view of what's actually happening in a reaction by focusing only on the species that undergo changes. While mastering how to write balanced chemical equations and their net ionic forms can seem daunting, using tools like a worksheet with answers can significantly demystify the process. Here, we'll explore the methodology behind net ionic equations, provide some examples, and discuss how these equations help chemists understand reactions at the molecular level.
Understanding Net Ionic Equations

A net ionic equation shows only the chemical species that are directly involved in the reaction. These equations exclude spectator ions, which are ions that remain unchanged throughout the reaction. By focusing on just the reactants and products that change, net ionic equations reveal the essence of the chemical transformation without the distraction of unchanged elements.
Basic Concepts

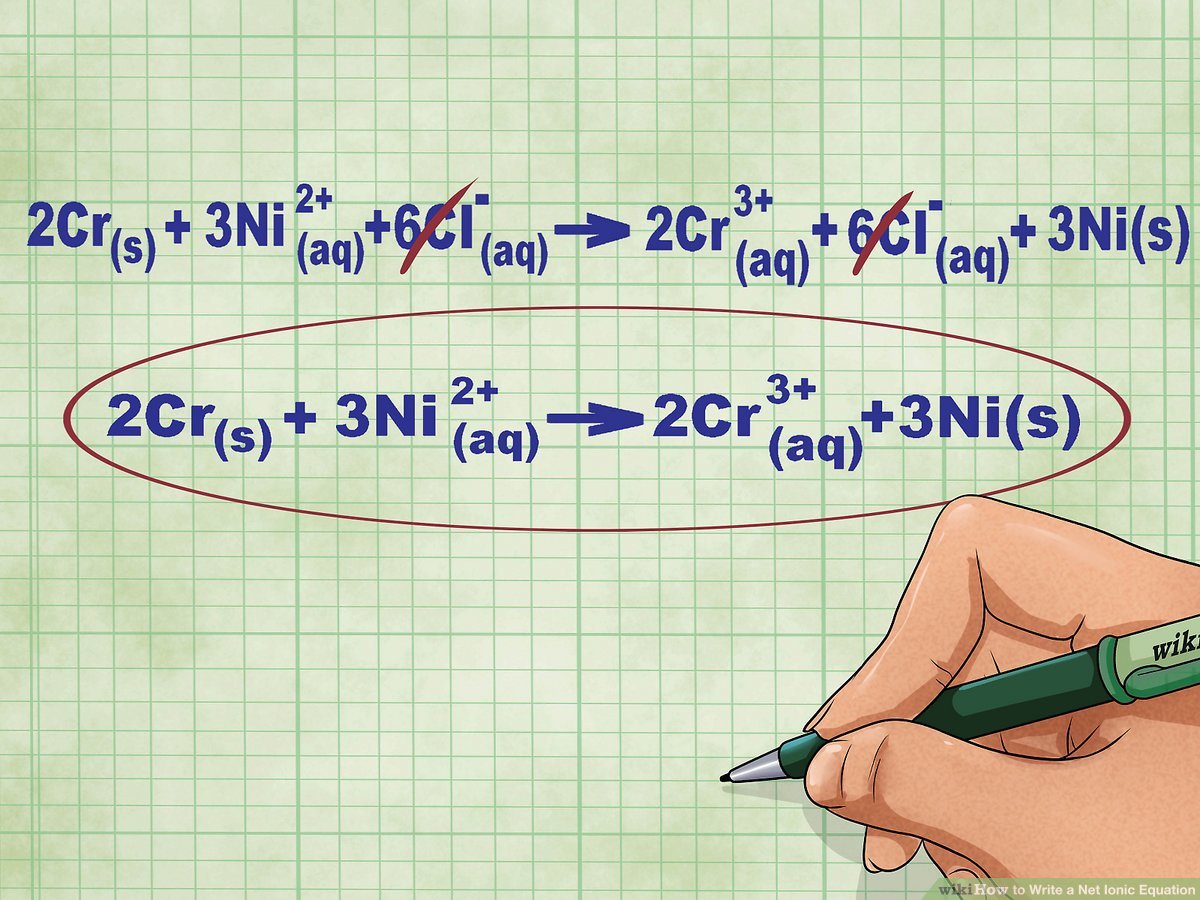
- Complete ionic equation: This shows all ions present in the solution at the beginning of the reaction.
- Spectator ions: These ions appear on both sides of the equation and do not participate in the reaction.
- Net ionic equation: By canceling out spectator ions from the complete ionic equation, we get the net ionic equation, which highlights only the entities that change.
Examples

Let’s go through an example:
- The reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl):
Complete ionic equation:
Na+(aq) + OH-(aq) + H+(aq) + Cl-(aq) → Na+(aq) + Cl-(aq) + H2O(l)
Net ionic equation:
H+(aq) + OH-(aq) → H2O(l)
⚠️ Note: Spectator ions are canceled out to focus on the actual reaction.
Worksheet with Answers

Here’s a simple worksheet to help you practice writing net ionic equations:
| Equation | Complete Ionic Equation | Net Ionic Equation |
|---|---|---|
| AgNO3 + NaCl → AgCl + NaNO3 | Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) → AgCl(s) + Na+(aq) + NO3-(aq) | Ag+(aq) + Cl-(aq) → AgCl(s) |
| BaCl2 + Na2SO4 → BaSO4 + 2 NaCl | Ba2+(aq) + 2 Cl-(aq) + 2 Na+(aq) + SO42-(aq) → BaSO4(s) + 2 Na+(aq) + 2 Cl-(aq) | Ba2+(aq) + SO42-(aq) → BaSO4(s) |
How to Use the Worksheet

- Write out the complete ionic equation, ensuring all ions are correctly represented.
- Identify and cancel out the spectator ions, which appear unchanged on both sides of the equation.
- Write down the net ionic equation after canceling the spectator ions.
The Importance of Net Ionic Equations

Net ionic equations provide several benefits:
- Simplicity: By focusing on the species that change, these equations are cleaner and easier to understand.
- Understanding reactivity: They reveal what is truly reacting in a solution, which helps in predicting the products of reactions.
- Environmental impact: Understanding the reactions at the ion level can inform how to reduce or eliminate harmful byproducts.
- Industrial applications: Chemists use net ionic equations to optimize industrial processes for better efficiency.
📘 Note: Writing net ionic equations isn't just academic; it's a fundamental skill in practical chemistry.
After dissecting the processes involved in net ionic equations through examples and a worksheet, it's clear how they streamline complex chemical reactions. These equations highlight the essence of chemical transformations, making it easier to predict outcomes, optimize processes, and understand environmental impacts. By stripping away the unchanged, net ionic equations give chemists a clearer, more direct insight into reactions at the molecular level.
Why are net ionic equations important in chemistry?

+
They simplify the understanding of complex reactions by focusing only on the species that undergo changes, aiding in the analysis of reaction outcomes and processes.
Can you explain what spectator ions are?

+
Spectator ions are ions that do not participate in the chemical reaction; they remain unchanged both before and after the reaction.
What is the difference between a complete ionic equation and a net ionic equation?

+
A complete ionic equation shows all ions present in the solution before and after the reaction. The net ionic equation removes the spectator ions, focusing only on the ions involved in the chemical change.
How can I determine if an ion is a spectator ion?

+
Look for ions that appear on both sides of the complete ionic equation; these ions do not change throughout the reaction and are therefore spectator ions.
Are there any tips for writing net ionic equations more efficiently?

+
Practice with a variety of reactions, keep track of common ions, and use solubility rules to predict which ions will precipitate or remain in solution. Also, focusing on species that change states or form a new compound can speed up the process.


