Naming Polyatomic Ions Made Easy: Worksheet Guide

Understanding chemistry can often seem like learning a foreign language, but the basics like naming polyatomic ions can be straightforward with the right tools and methods. This comprehensive guide will walk you through the process of easily identifying and naming these common chemical entities, making your journey into chemistry a bit more manageable.
What Are Polyatomic Ions?

Polyatomic ions are groups of atoms that carry an electric charge but still move together as a single unit. They are crucial in various chemical reactions, especially in the formation of salts. Here’s how to spot and name them:
- Sulfate: SO42- - A polyatomic ion that commonly forms sulfates when combined with various metals.
- Nitrate: NO3- - Frequently involved in plant nutrition, especially in fertilizers.
- Phosphate: PO43- - Vital in biology for energy transfer, as in ATP.
How to Recognize Polyatomic Ions

When learning to recognize polyatomic ions, here are a few things to keep in mind:
- Endings: Many polyatomic ions have characteristic endings like -ate, -ite, -ide.
- Charge: They carry a distinct electrical charge, either positive or negative.
- Formulas: Learning their formulas is crucial as they do not dissociate into individual ions.
Naming Polyatomic Ions

Here’s how to correctly name polyatomic ions:
1. Look at the Elements
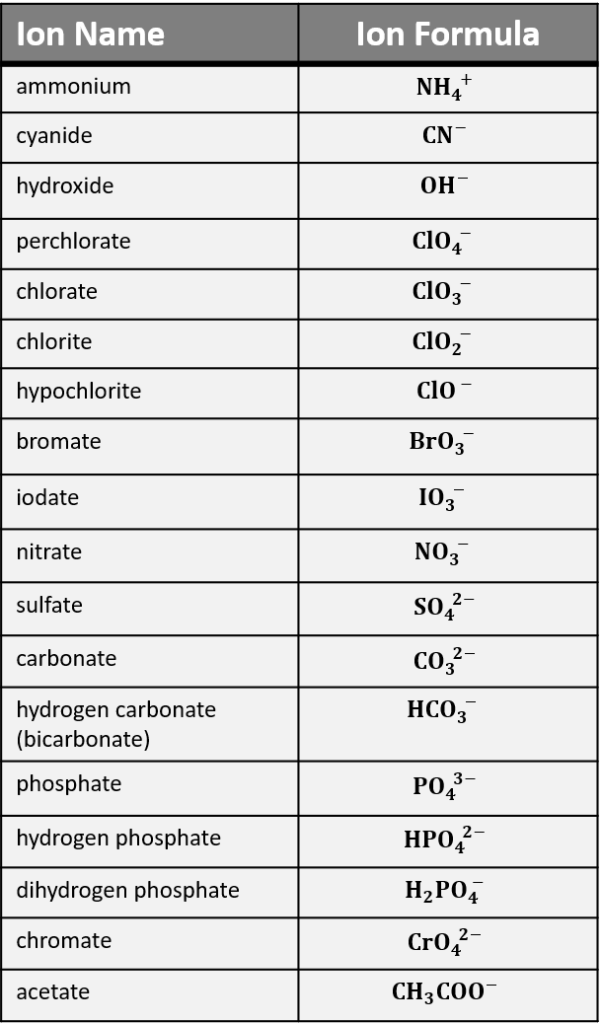
Polyatomic ions often include common elements like oxygen, hydrogen, nitrogen, sulfur, or phosphorus:
- Nitrogen ions:
- NH4+: Ammonium ion
- NO3-: Nitrate ion
- Oxygen ions:
- OH-: Hydroxide ion
- CO32-: Carbonate ion
2. Use the Ending Rules

When an element is combined with oxygen:
- -ate suffix for the common oxidation state.
- -ite suffix when there is one less oxygen atom than in the -ate form.
- -ide suffix for ions with no oxygen.
3. Combine Names

If a polyatomic ion has more than one name, combine them like so:
- Sulfite and hyposulfite (for SO32-)
Here is a table to illustrate some polyatomic ions:
| Formula | Name | Charge |
|---|---|---|
| SO42- | Sulfate | 2- |
| NO2- | Nitrite | 1- |
| ClO2- | Chlorite | 1- |

4. Prefixes for Variation

To differentiate ions with similar compositions:
- Per- means one more oxygen atom than -ate.
- Hypo- means one less oxygen atom than -ite.
Tips for Learning Polyatomic Ions

Making learning easier:
- Flashcards: Create or find flashcards for memorizing names, formulas, and charges.
- Patterns: Look for patterns in the names and formulas.
- Mnemonics: Use mnemonics to remember unique ions.
Integrating Polyatomic Ions in Chemical Nomenclature

Here’s how to use polyatomic ions in naming compounds:
- Salts: Combine metal ions with polyatomic anions, like NaNO3 (sodium nitrate).
- Acids: Use prefixes like 'hydro-' for acids without oxygen, and suffixes for oxygen-containing acids.
- Neutral Compounds: Balance charges to name compounds, e.g., (NH4)2SO4 (ammonium sulfate).
👉 Note: Always cross-reference the ions' charges to ensure the compound is neutral or correctly charged.
To summarize, naming polyatomic ions doesn't have to be daunting. With practice, pattern recognition, and understanding the rules of naming, you'll master this aspect of chemistry in no time. The key is to approach it systematically, use memory aids, and get lots of practice. As you become more comfortable with these ions, chemical nomenclature will become much clearer, making your study of chemistry more rewarding.
Why are polyatomic ions important in chemistry?

+
Polyatomic ions are important because they play a crucial role in many chemical reactions, including acid-base reactions, oxidation-reduction reactions, and the formation of complex ions and salts.
What’s the difference between -ate and -ite in polyatomic ions?

+
The -ate suffix is used for polyatomic ions that contain the common oxidation state of an element, while -ite indicates one less oxygen atom than in the -ate form.
Can polyatomic ions dissociate in solutions?

+
Polyatomic ions can exist in solution as a single unit, but when salts dissolve, they dissociate into their constituent ions, including polyatomic ones.
How do I remember all the polyatomic ions?

+
Memory aids like flashcards, songs, or mnemonics can help, and understanding the patterns in their names and charges will make memorization easier.



