5 Essential Tips for Naming Ionic Compounds Correctly

Understanding how to name ionic compounds correctly is a cornerstone of learning chemistry. Ionic compounds are chemical compounds composed of ions held together by electrostatic forces termed ionic bonding. Given their widespread use in various fields like pharmaceuticals, agriculture, and materials science, accurately naming them ensures proper communication among professionals and researchers. Here are five essential tips to guide you through the process of naming ionic compounds accurately.
Understand the Basics of Ionic Compound Nomenclature

The first step in naming ionic compounds involves understanding the basics:
- Ionic compounds consist of positively charged cations and negatively charged anions.
- The name typically consists of the cation followed by the anion.
- The cation can be from metals, which form positive ions by losing electrons, or polyatomic ions (like ammonium).
- Anions are usually from non-metals or polyatomic ions, gaining electrons to form negative ions.

Name the Cation

The cation's name is relatively straightforward:
- Metals: Use the element's name directly (e.g., Sodium for Na+).
- Transition Metals: Specify the charge with Roman numerals in parentheses (e.g., Iron(II) for Fe2+).
- Polyatomic Ions: Use the common name (e.g., Ammonium for NH4+).
🔎 Note: Some metals like Zinc or Silver typically only have one common ionic charge, so Roman numerals are not needed.
Name the Anion
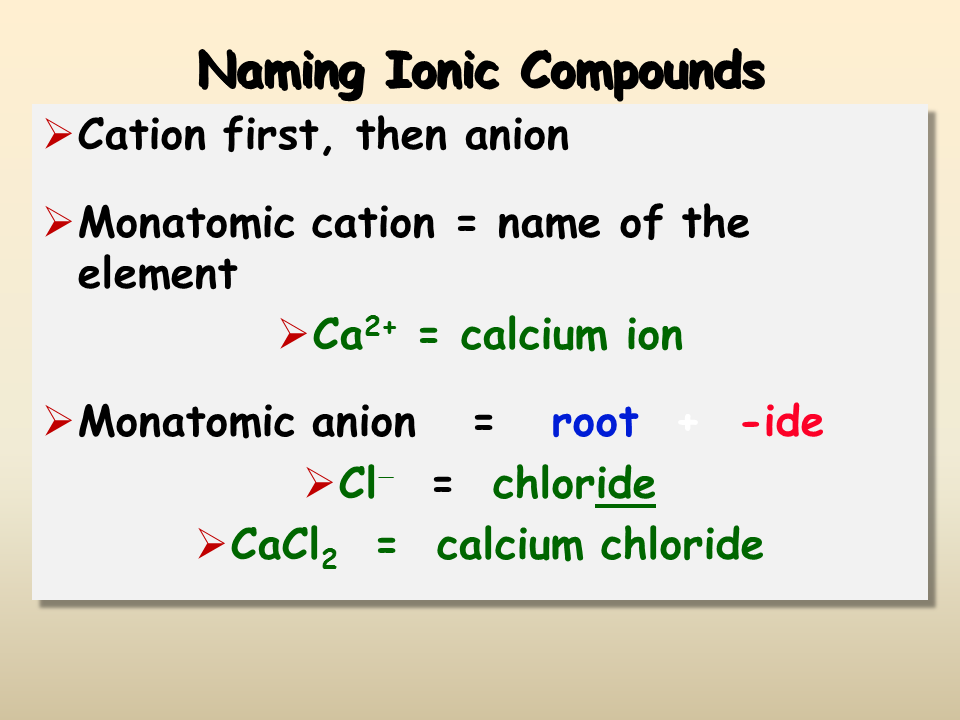
The anion's naming conventions vary:
- Monoatomic Anions: Change the ending of the element's name to -ide (e.g., Chlorine becomes Chloride for Cl-).
- Polyatomic Anions: Generally retain their names with adjustments for suffixes if necessary (e.g., Nitrate for NO3-).

Combine Names Correctly

Here’s how you put it all together:
| Compound | Cation | Anion | Correct Name |
|---|---|---|---|
| NaCl | Sodium (Na+) | Chloride (Cl-) | Sodium Chloride |
| FeO | Iron(II) (Fe2+) | Oxide (O2-) | Iron(II) Oxide |
| CaSO4 | Calcium (Ca2+) | Sulfate (SO42-) | Calcium Sulfate |

Watch Out for Hydrates

Ionic compounds can sometimes contain water molecules in their crystal lattice known as hydrates. Here's how you handle them:
- Identify the hydrate by its dot and number (e.g., CuSO4·5H2O).
- Name the anhydrous compound, then add the number of water molecules followed by the word "hydrate."
Example: CuSO4·5H2O would be named Copper(II) Sulfate Pentahydrate.

In summary, correctly naming ionic compounds involves understanding the nature of ions involved, following established conventions for cation and anion names, and properly combining these names. Recognizing special cases like hydrates and being aware of common polyatomic ions also enhances accuracy in your nomenclature. Remember, accurate naming is crucial for clear communication and application in various scientific and industrial fields.
How do I name a compound with transition metals?

+
For transition metals, use Roman numerals to indicate the charge of the cation. For example, FeCl2 would be Iron(II) Chloride, where the Roman numeral (II) specifies the iron ion’s +2 charge.
What is the difference between -ite and -ate in polyatomic ions?

+
The suffix -ite denotes one less oxygen atom than the -ate version of the same polyatomic ion. For example, SO32- is sulfite while SO42- is sulfate.
How do I name an ionic compound that includes a polyatomic ion?

+
Name the cation first, then the polyatomic anion. For example, KNO3 is named Potassium Nitrate.
What happens if an ionic compound contains more than one type of cation?

+
If an ionic compound has more than one type of cation, you must specify the amount of each cation present using Greek prefixes (mono-, di-, tri-, etc.) along with the cation’s charge. For example, (NH4)2SO4 would be named Ammonium Sulfate.
Why do some metals not need Roman numerals for naming?

+
Some metals like sodium, calcium, and magnesium only form one type of cation with a predictable charge, making Roman numerals unnecessary.