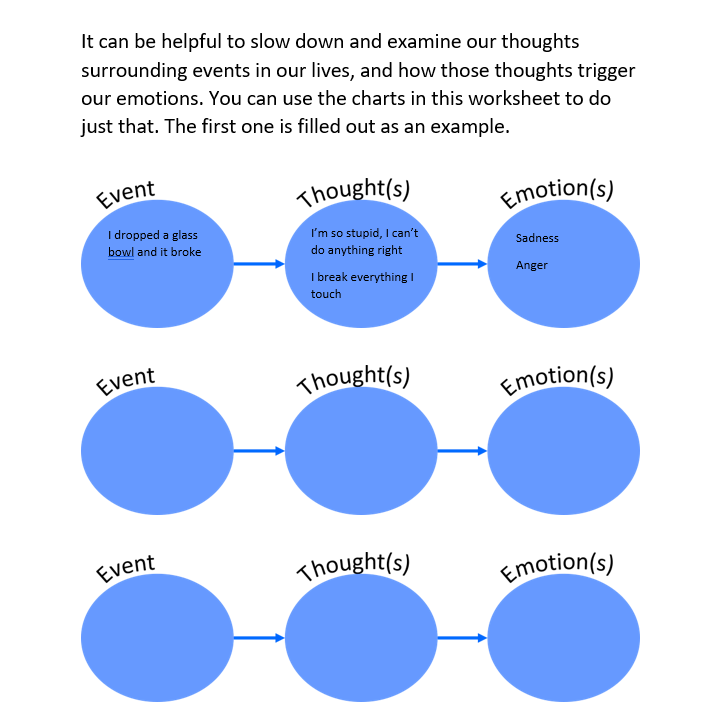
5 Essential Answers for Naming Ionic Compounds

In the world of chemistry, understanding how to name compounds correctly is fundamental. Ionic compounds, formed by the electrostatic attraction between oppositely charged ions, often pose a challenge due to their complexity. Whether you're a student delving into the basics of chemistry, a teacher explaining these concepts, or a researcher looking for clarity, mastering the rules for naming ionic compounds is crucial. This post will walk you through the essentials, ensuring you can name ionic compounds with confidence.
Why Naming Compounds is Important
The process of naming compounds isn’t just a matter of academic exercise; it has real-world implications:
- Safety: Correct identification ensures safety in handling chemicals, preventing potentially hazardous reactions or confusion in a laboratory or industrial setting.
- Communication: Standardized names allow for clear and unambiguous communication among scientists and researchers globally.
- Understanding: Proper nomenclature helps in predicting properties, understanding chemical reactions, and facilitating the study of chemical behavior.
⚠️ Note: Incorrect naming can lead to confusion, errors in experiments, or misinterpretation of data.
Understanding Ionic Bonds

An ionic bond is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions. Here’s a brief overview:
- Ions: Atoms or groups of atoms that have a positive or negative charge due to the gain or loss of electrons.
- Ionic Bonding: When one element loses an electron(s) to another, forming positive (cation) and negative (anion) ions.
- Examples: Sodium chloride (NaCl), magnesium oxide (MgO), and potassium bromide (KBr) are typical ionic compounds.

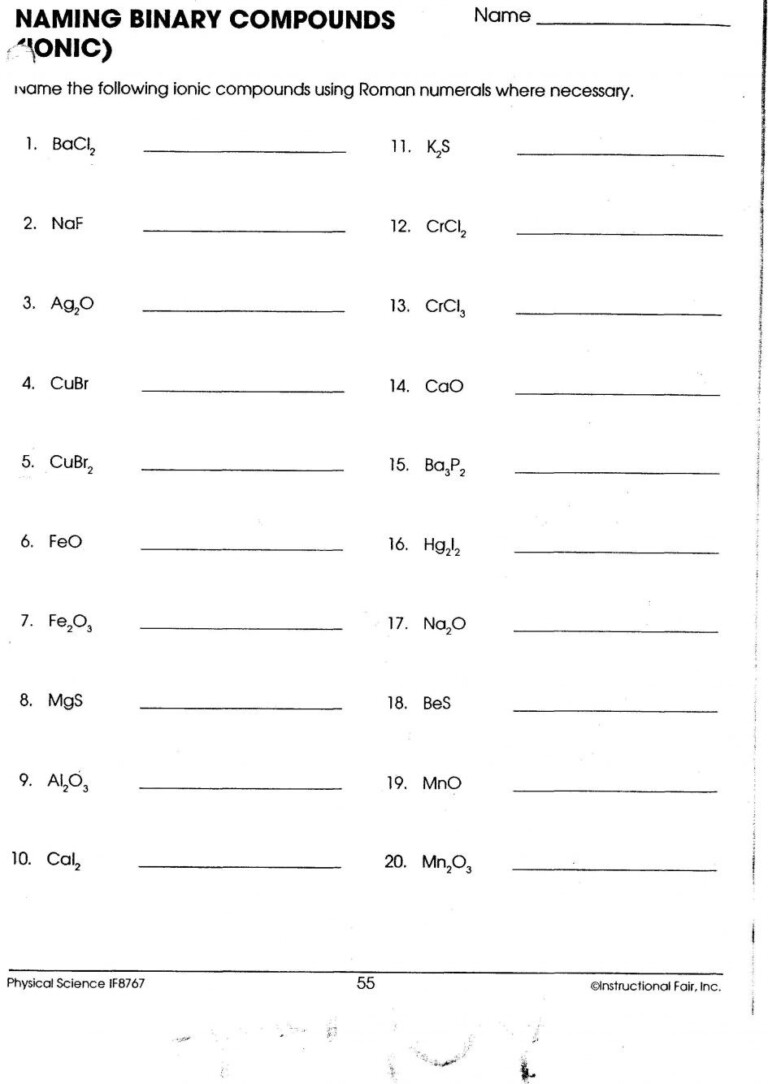
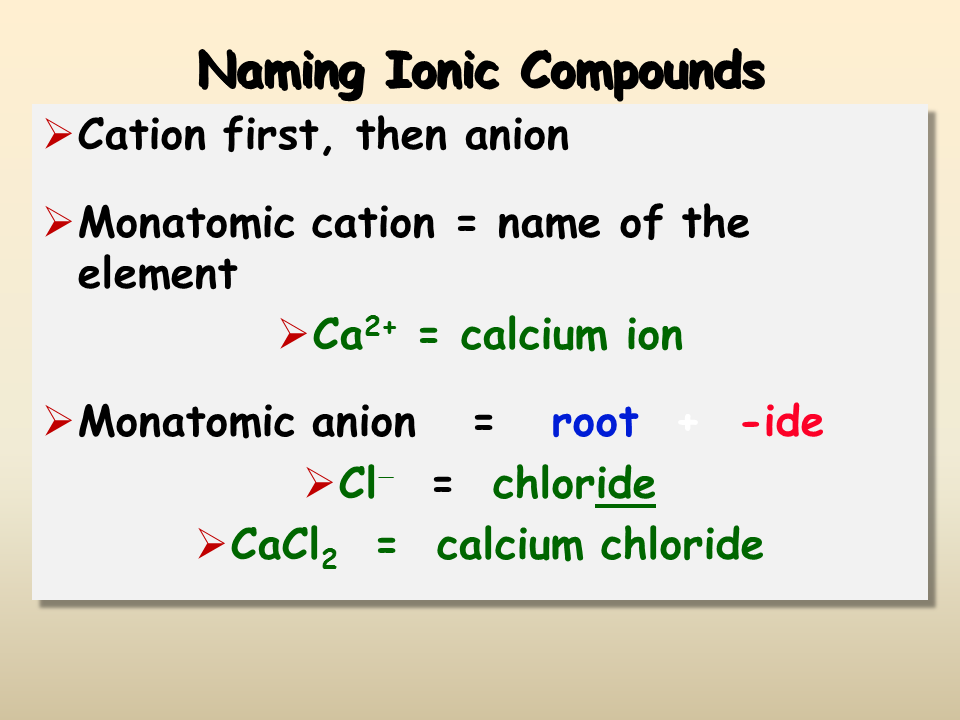
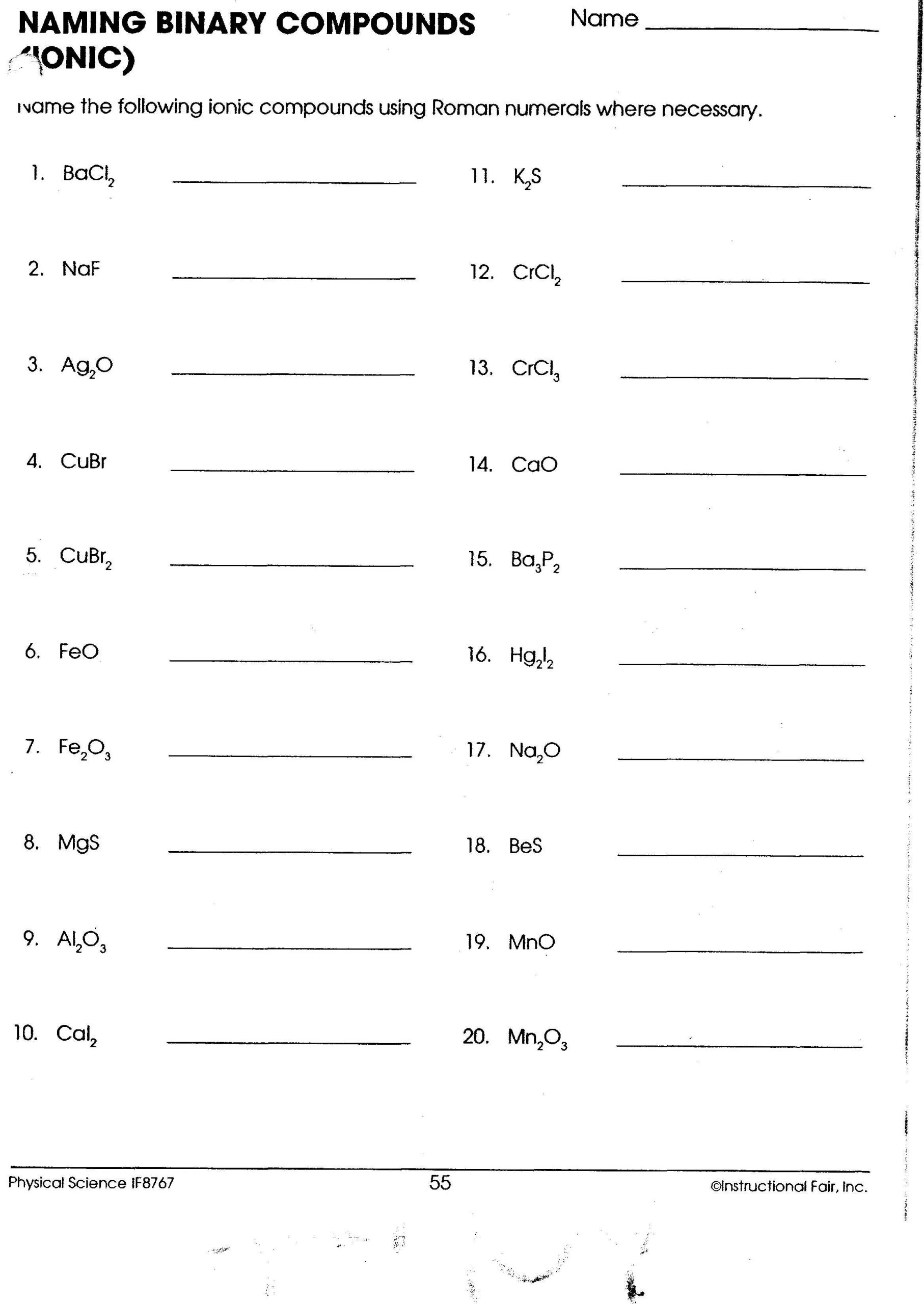
Rules for Naming Ionic Compounds

Here are the basic rules to follow when naming ionic compounds:
- First Name the Metal: Use the element name of the cation without any change.
- Then Name the Non-Metal: Change the ending of the anion element name to “-ide.”
- Specify the Charge: When a metal can form more than one cation, use Roman numerals in parentheses to indicate the charge.
- Polyatomic Ions: For polyatomic ions, simply use their given name from the chart of common polyatomic ions.
Example:

Take sodium chloride as an example:
- The cation, sodium, is written first as sodium.
- The anion, chlorine, becomes chloride.
- The full name: Sodium chloride.
💡 Note: Remember, for transition metals with variable charges, Roman numerals are crucial in clarifying which cation is involved.
Handling Variable Charge Metal Ions
Some metals can form more than one type of ion. Here’s how to deal with this:
- Roman Numerals: Use the Stock system where a Roman numeral in parentheses indicates the cation’s charge, e.g., Fe(II) for iron(II) ion or Fe(III) for iron(III) ion.
- Old System: The classical or Latin system uses suffixes (ous for lower charge, -ic for higher charge), e.g., cuprous oxide (Cu₂O) and cupric oxide (CuO).
Example:

Consider copper (II) oxide:
- Copper can have a +1 or +2 charge, so we use a Roman numeral.
- The oxide anion remains as “oxide.”
- Full name: Copper(II) oxide.
🔎 Note: Both systems can be used, but the Stock system is preferred in modern chemistry.
Dealing with Polyatomic Ions

Polyatomic ions are groups of atoms with an overall charge:
- Common Ions: Learn common polyatomic ions such as sulfate (SO₄²⁻), nitrate (NO₃⁻), and carbonate (CO₃²⁻).
- Naming Rules: Use the name of the polyatomic ion directly in the compound’s name.
| Polyatomic Ion | Formula |
|---|---|
| Sulfate | SO₄²⁻ |
| Nitrate | NO₃⁻ |
| Carbonate | CO₃²⁻ |

Example:

Name potassium nitrate:
- Potassium is the cation.
- Nitrate is the polyatomic anion.
- Full name: Potassium nitrate.
Common Pitfalls and How to Avoid Them

Here are some common errors in naming ionic compounds:
- Misidentifying Polyatomic Ions: Ensure you know common polyatomic ions and their charges to prevent confusion.
- Forgetting Roman Numerals: Always use Roman numerals for transition metals with variable charges.
- Confusing Ionic and Molecular Compounds: Ionic compounds contain metal ions, while molecular compounds usually contain non-metals. Make this distinction clear.
By following these rules, you'll be able to name ionic compounds accurately. Remember, consistency in naming leads to better understanding and communication in chemistry. The ability to name compounds correctly is a skill that not only helps in academic pursuits but also has practical applications in various scientific and industrial fields.
To sum up, knowing how to name ionic compounds correctly involves understanding the charge of ions, recognizing polyatomic ions, and applying basic naming rules. This foundational knowledge ensures safety, accuracy, and effective communication in the chemical community, enabling you to navigate the complexities of chemistry with ease.
What is an ionic bond?

+
An ionic bond is formed by the electrostatic attraction between oppositely charged ions, typically a metal cation and a non-metal anion.
Why use Roman numerals in the names of some ionic compounds?

+
Roman numerals are used to specify the charge of the metal ion when it can form multiple ions with different charges, ensuring clarity in the compound’s name.
How do you know if an ion is polyatomic?

+
Polyatomic ions are groups of atoms bonded together that carry a net charge, commonly known names include sulfate, nitrate, and phosphate.
What are the differences between ionic and covalent bonds?
+
Ionic bonds involve the transfer of electrons, creating charged ions, whereas covalent bonds involve the sharing of electrons between atoms, resulting in no net charge on the molecules.