5 Tips for Naming Compounds with Polyatomic Ions

Understanding how to name compounds that contain polyatomic ions is a fundamental skill in chemistry. These ions, which are groups of atoms that behave as a single unit when forming compounds, can be particularly challenging for students. This post will explore effective strategies for naming these compounds, ensuring clarity and accuracy in chemical notation.
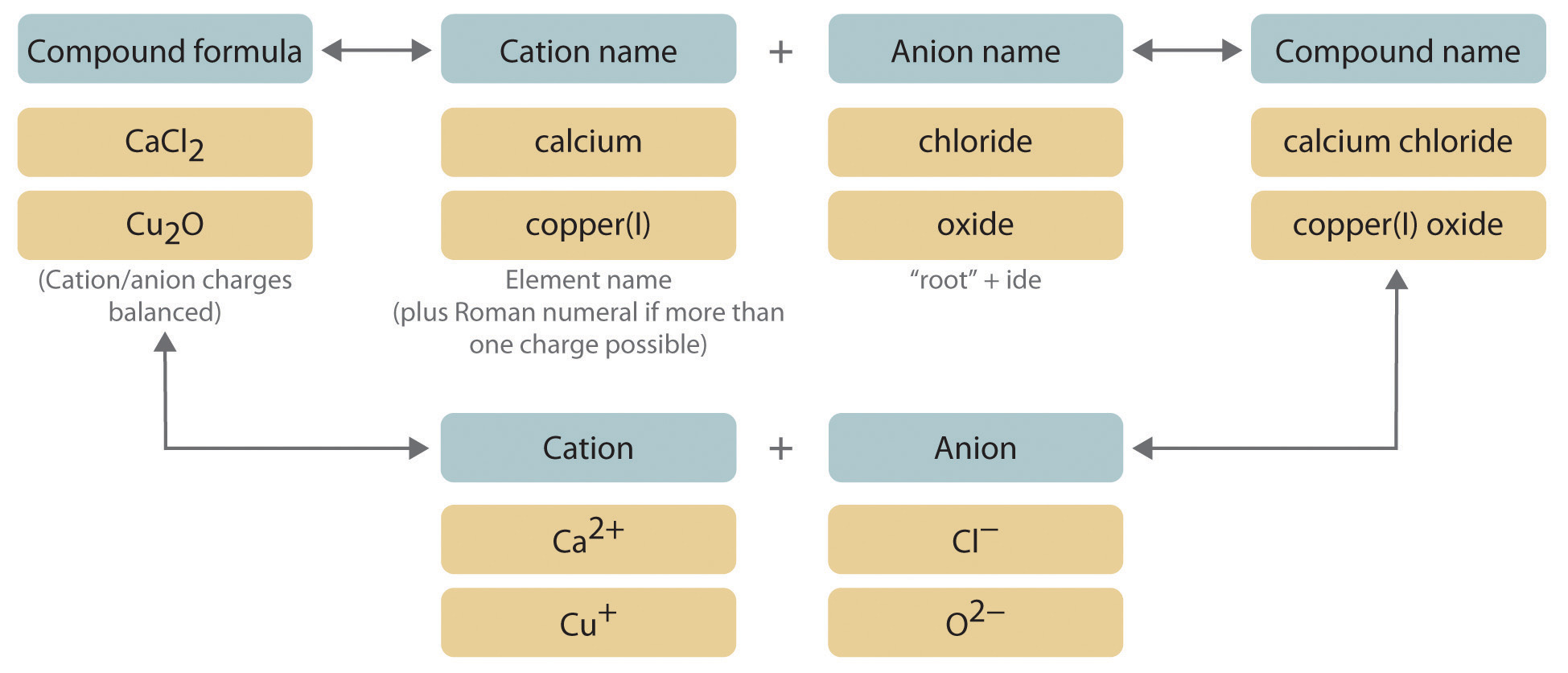
Identify the Polyatomic Ion

The first step in naming compounds with polyatomic ions is to identify the ion itself. Here are key points to consider:
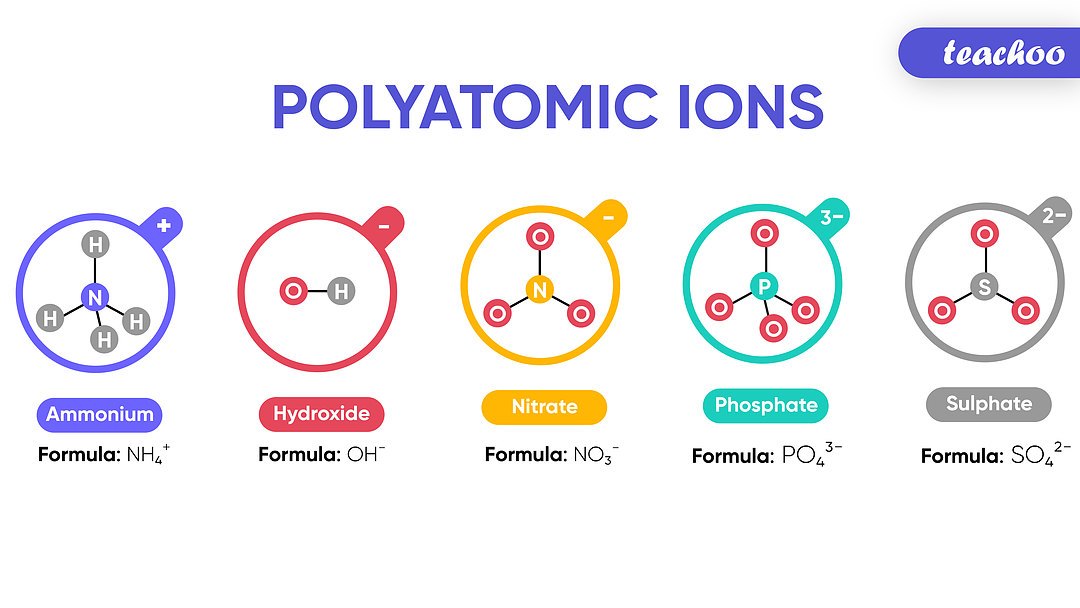
- Common Ions: Familiarize yourself with common polyatomic ions like ammonium (NH4+), sulfate (SO42-), and nitrate (NO3-). There are typically around 20 that are encountered frequently.
- Charge: Each polyatomic ion has a specific charge, which will dictate how it bonds with other ions or elements.
- Table of Polyatomic Ions: Here is a basic table for reference:
| Polyatomic Ion | Formula | Charge |
|---|---|---|
| Ammonium | NH4+ | +1 |
| Carbonate | CO32- | -2 |
| Nitrate | NO3- | -1 |
Knowing these common polyatomic ions will significantly ease the naming process.
Charge Balancing

Once you’ve identified the polyatomic ion, ensure the charges balance out with the other ion or element:
- The total positive charge must equal the total negative charge. For example, in ammonium sulfate [(NH4)2SO4], two ammonium ions each with a +1 charge are needed to balance one sulfate ion with a -2 charge.
📝 Note: Remember, the positive and negative charges must be equal in absolute value for the compound to be electrically neutral.
Using Roman Numerals (Transition Metals)

When dealing with transition metals, which can have various charges, use Roman numerals to indicate the metal’s charge:
- For example, Iron(III) sulfate would indicate iron has a +3 charge, matching the -2 charge of sulfate.
📝 Note: This method is known as the Stock system, and it’s crucial for accurately naming compounds involving transition metals.
Prefixes for Binary Molecular Compounds
While not always associated with polyatomic ions, understanding prefixes is useful, particularly if the compound includes a polyatomic ion in its molecular form:
- Mono-, di-, tri-, tetra-, penta-, etc., are used to denote the number of each atom present.
- Examples include carbon dioxide (CO2) and dinitrogen pentoxide (N2O5).
Practice and Memorization

Lastly, mastering the naming of compounds with polyatomic ions requires practice:
- Memorize common polyatomic ions and their charges.
- Practice balancing charges in different compounds.
- Use flashcards or online quizzes to improve retention of this information.
📝 Note: There are no shortcuts to learning these; repetition and application are key to success.
In summary, naming compounds with polyatomic ions involves identifying the polyatomic ion, balancing charges, applying Roman numerals when necessary, understanding prefixes, and consistent practice. Each of these tips serves to clarify the complexity and precision required in chemical nomenclature. With these tools in your chemistry toolkit, you'll be well-prepared to handle any compound naming challenge with confidence.
Why is it important to identify polyatomic ions in compounds?

+
Identifying polyatomic ions is crucial because these ions often have unique properties and reactions that differ from simple monatomic ions, affecting how the compound behaves and interacts.
What if I don’t recognize a polyatomic ion?

+
If you encounter an unfamiliar polyatomic ion, consult a reference list or periodic table that lists common polyatomic ions with their charges. You can also deduce the ion’s identity based on the compound’s chemical formula and the charges of other known ions present.
How do I know when to use Roman numerals in naming?

+
Roman numerals are used when naming compounds with transition metals, which can have multiple oxidation states. The numeral indicates the metal’s charge, which must match the total charge of the accompanying polyatomic ion or ions.



