5 Easy Tips for Naming Binary Covalent Compounds

Mastering the art of naming chemical compounds, particularly binary covalent compounds, is an essential skill for chemistry students. Binary covalent compounds consist of two non-metal elements bonded together with covalent bonds. These compounds can be tricky to name due to the need to adhere to certain rules and conventions. In this comprehensive guide, we will explore five straightforward tips to help you master the naming of binary covalent compounds effortlessly.
1. Identify the Elements Present
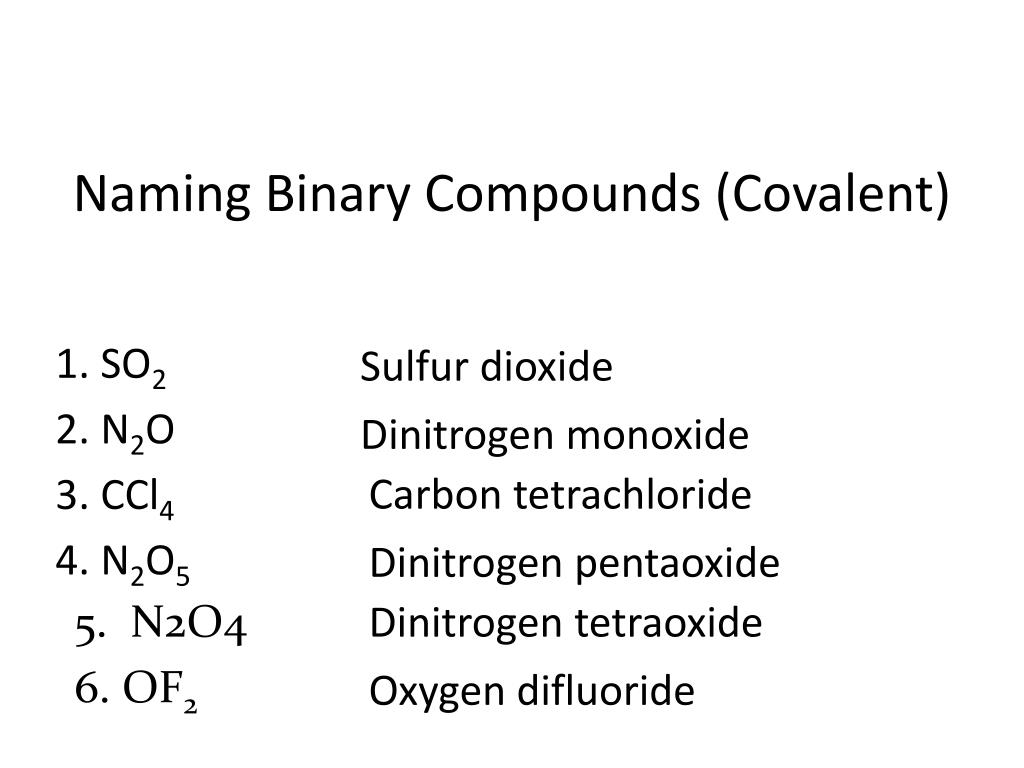
Before you can name a binary covalent compound, you need to know which elements are involved. Always check the chemical formula to determine:
- The type of elements present - Are they both non-metals?
- The placement of the elements in the periodic table - What are their respective groups?
🔍 Note: Remember that binary covalent compounds are formed between two non-metals. If you see elements like carbon, hydrogen, nitrogen, oxygen, phosphorus, sulfur, or halogens paired together, you're dealing with a binary covalent compound.
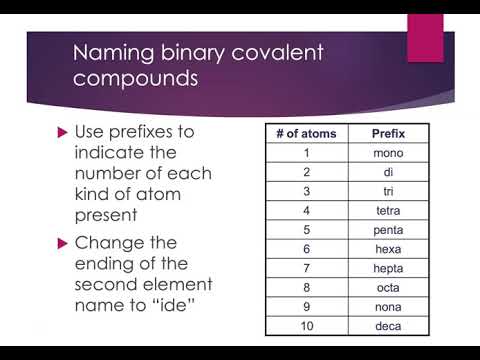
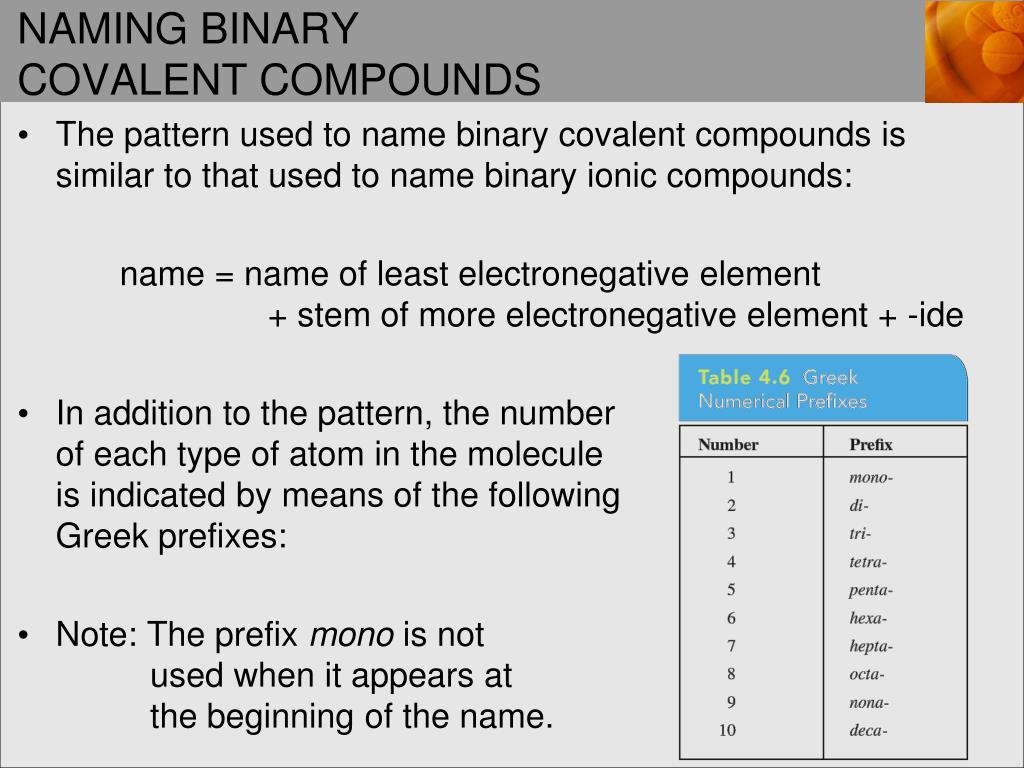
2. Use Prefixes for Multiple Atoms
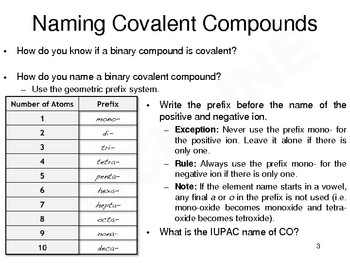
When naming binary covalent compounds, prefixes are used to indicate the number of atoms of each element present in the molecule. Here’s the list of common prefixes:
| Number of Atoms | Prefix |
|---|---|
| 1 | mono- |
| 2 | di- |
| 3 | tri- |
| 4 | tetra- |
| 5 | penta- |
| 6 | hexa- |
| 7 | hepta- |
| 8 | octa- |
| 9 | nona- |
| 10 | deca- |

Always drop the ‘o’ or ‘a’ from the prefix when the second element’s name starts with a vowel.
✏️ Note: The first element in the formula does not use a prefix if it has only one atom; this means you do not write 'mono-' for the first element unless there is more than one atom of it.
3. Order the Elements Correctly
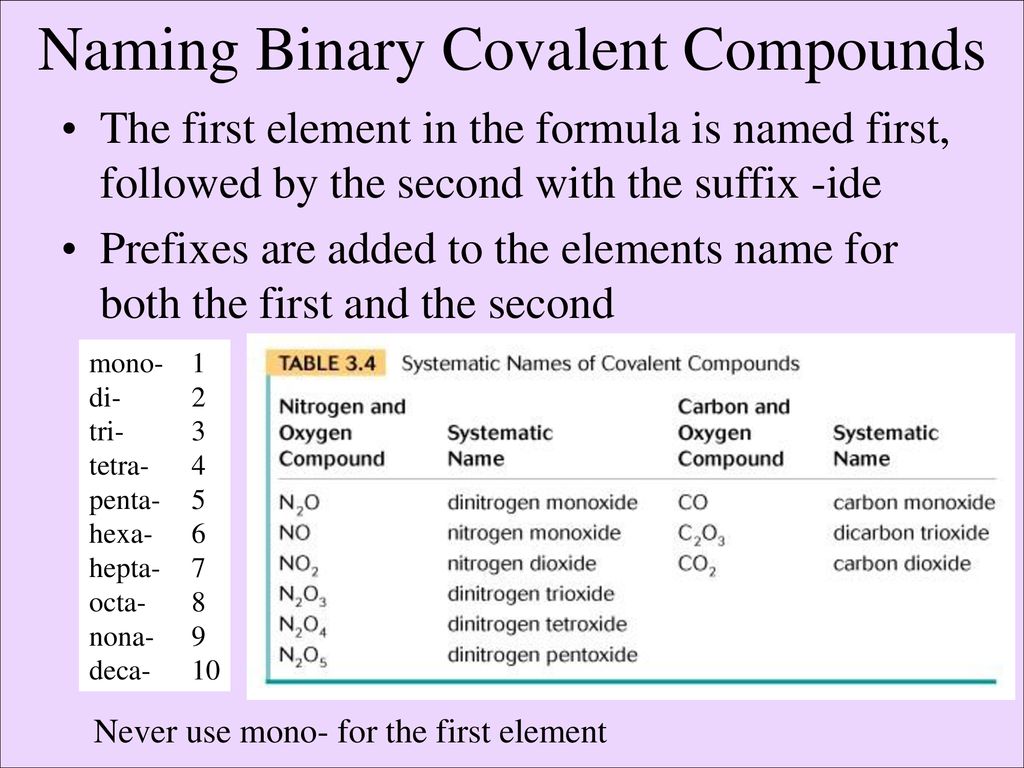
The order of elements in the name of a binary covalent compound follows a specific pattern:
- List the less electronegative element first.
- The more electronegative element comes second, and its name ends in the suffix “-ide.”
📚 Note: This rule ensures consistency in chemical nomenclature, allowing chemists to communicate effectively about compounds without ambiguity.
4. Combine Prefixes and Element Names
Now, put together the prefixes with the element names:
- Write the name of the first element followed by its numerical prefix (if more than one).
- Follow with the stem of the second element’s name and add “-ide” with its numerical prefix.
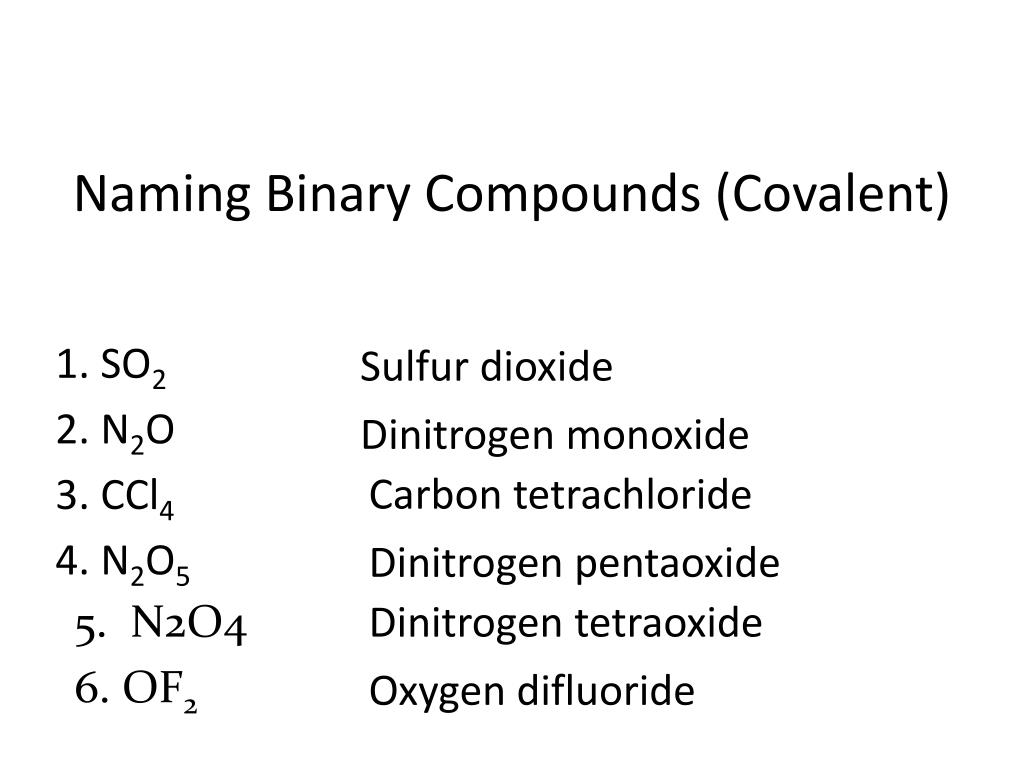
For example, CO2 is named carbon dioxide (one carbon, two oxygens).
5. Exceptions and Common Compounds

Not all binary covalent compounds follow the prefix system strictly:
- Compounds with common names like water (H2O), ammonia (NH3), or methane (CH4) are often just referred to by these names, rather than their systematic names.
- Some compounds might also have a traditional name that’s widely used alongside or instead of their systematic name. For instance, H2O is also known as dihydrogen monoxide in some contexts.
🌐 Note: In real-world applications, the historical or traditional names can be just as important as the systematic ones, especially in industry or common usage.
To summarize, successfully naming binary covalent compounds involves:
- Identifying the elements and their quantities within the compound.
- Using appropriate numerical prefixes.
- Ordering the elements based on their electronegativity.
- Applying the suffix "-ide" to the second element.
- Keeping in mind common exceptions to the rules.
These tips should give you a solid foundation in naming binary covalent compounds. By practicing with real-world examples, you'll not only remember the process but also understand the underlying reasons behind chemical nomenclature. Whether you're in a chemistry class or working in a lab, this knowledge will help you communicate effectively and avoid confusion when dealing with these essential compounds.
What if a binary covalent compound does not use prefixes?
+
Some binary covalent compounds have traditional or common names that do not strictly follow the prefix system. For instance, water (H₂O) is known universally by its common name, not its systematic name, dihydrogen monoxide. Always remember, common usage can trump systematic naming, especially for historical or well-known compounds.
How do I remember the numerical prefixes for naming?

+
Learning the prefixes can be fun by associating them with words or objects. For example, ‘di’ sounds like ‘die,’ which you might link with ‘2’ (two dice); ‘tri’ can remind you of a tripod (three legs); ‘tetra’ is like tetrapod (four legs). Use mnemonic devices or create stories to make the learning process more engaging.
Can binary covalent compounds have ionic bonds?
+
No, binary covalent compounds are formed exclusively between two non-metals, and they share electrons rather than transfer them, which is characteristic of ionic bonding. Covalent compounds involve covalent bonds where electrons are shared between atoms to achieve stability.



