Bohr Model Diagrams: 5 Simple Steps for Students

In the fascinating world of atomic physics, the Bohr Model offers a simplified way to visualize atomic structure. This model, introduced by Niels Bohr, provides a clear framework for understanding how electrons are arranged around the nucleus in various energy levels or shells. For students learning about atomic theory, mastering the Bohr Model can be instrumental in grasping the basics of chemistry and physics. Here, we guide you through 5 simple steps to create and understand Bohr Model Diagrams, making the abstract concepts of atomic structure accessible and visual.
Step 1: Understanding the Nucleus

The nucleus is the heart of an atom, and in the Bohr Model, it’s where you start your diagram.
- Identify the element’s atomic number, which indicates the number of protons in the nucleus.
- Include the same number of neutrons for simple models or find the specific neutron number for a more accurate representation.
🔍 Note: For simplicity, use the atomic number to determine the proton count. The number of neutrons often varies, creating isotopes, but for basic models, equal protons and neutrons is a common practice.
Step 2: Drawing Electron Shells

Once you’ve drawn the nucleus, it’s time to illustrate the electron shells.
- The first shell can hold up to 2 electrons, while the second holds up to 8, and the third 18, following the 2n² rule where n is the shell number.
- Start filling electrons from the innermost shell outward, placing each electron in a circular orbit around the nucleus.
Remember to keep the electrons equidistant from the nucleus within their respective shells.
Step 3: Placing the Electrons

With the shells in place, you’ll distribute the electrons:
- Fill the first shell (K shell) with up to 2 electrons.
- Continue filling the second shell (L shell) up to 8 electrons, and so on.
- Use dots or small circles to represent electrons in their respective orbits.
Step 4: Visual Representation
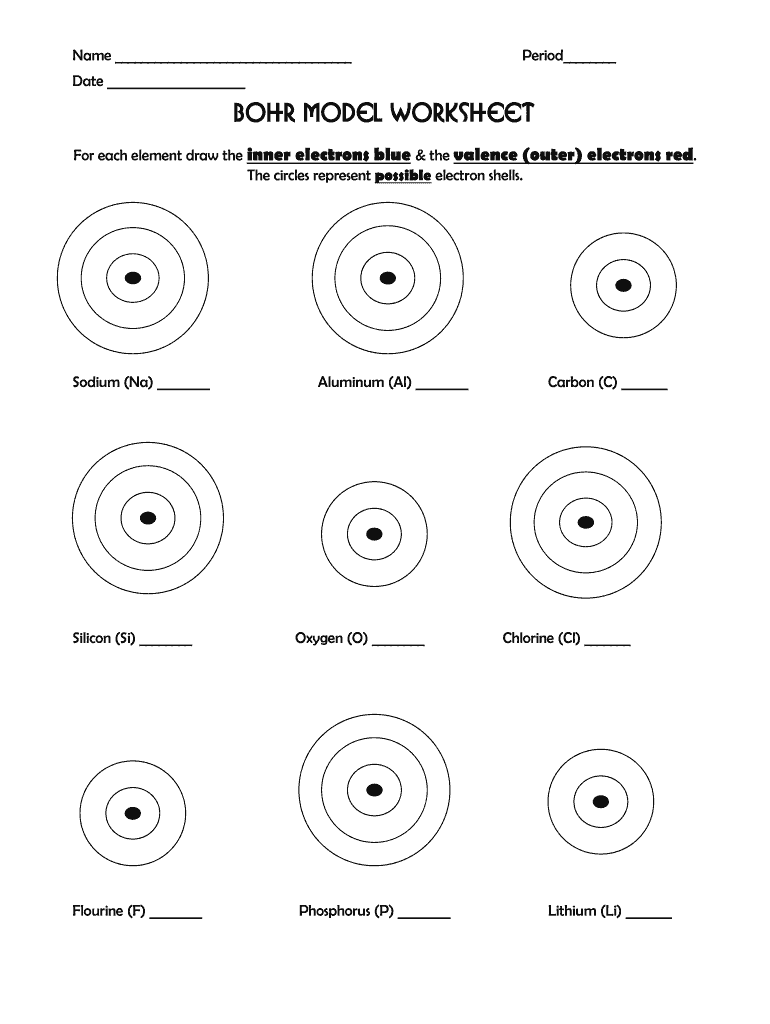
Now, let’s focus on making the model visually appealing and accurate:
- Choose a consistent scale for the size of protons, neutrons, and electrons relative to each other.
- Label each shell with its letter (K, L, M, etc.) for clarity.
Step 5: Enhancing the Diagram

Here are some enhancements you can make:
- Color code protons (positively charged) and electrons (negatively charged) for visual distinction.
- Add arrows to indicate electron movement or energy transition.
Creating Bohr Model Diagrams is not just about drawing; it's about understanding atomic structure. By following these steps, you can visualize how electrons are arranged in energy levels, and their potential movements, which are foundational concepts in atomic physics and chemistry. This model simplifies a complex system, making it accessible for students at various educational levels to understand and appreciate the atomic world.
📚 Note: While the Bohr Model is a simplification and has limitations, it's a great starting point for learning about atoms. More advanced models like quantum mechanics offer a deeper but more complex understanding.
Why is the first shell limited to 2 electrons in the Bohr Model?

+
The first electron shell, or K shell, has only one orbital (s orbital), which can hold a maximum of 2 electrons according to the quantum mechanical principle. This limitation stems from the principles of quantum mechanics, where the number of electrons an orbital can hold is determined by the formula 2(2l+1), where l is the angular momentum quantum number.
Can you show electrons jumping between orbits in a Bohr Model Diagram?

+
Yes, electrons can be depicted as moving between orbits in a Bohr Model to represent energy transitions. When an electron gains energy, it moves to a higher energy level, and when it loses energy, it falls to a lower one. This movement can be shown with arrows.
How accurate is the Bohr Model compared to modern atomic models?

+
The Bohr Model is a simplified version of atomic structure that was groundbreaking in its time but has limitations. Modern quantum mechanical models describe electron behavior more accurately by considering wave-like properties and probability distributions, offering a more nuanced view of electron orbitals and atomic structure.



