5 Essential Tips for Naming Chemical Formulas

The world of chemistry is rich with fascinating compounds, each with its unique properties and applications. A significant part of understanding chemistry involves correctly naming chemical compounds, which can be a daunting task for many students and professionals alike. This article aims to provide a clear guide on naming chemical formulas effectively, ensuring accuracy and consistency in scientific communication.
The Importance of Correct Chemical Naming
Naming chemical compounds accurately is not just a formality; it’s crucial for:
- Safety in handling and storage of chemicals.
- Clarity in scientific research and publications.
- Regulatory compliance in industries like pharmaceuticals, food, and environmental science.
- Academic assessment and understanding in educational settings.

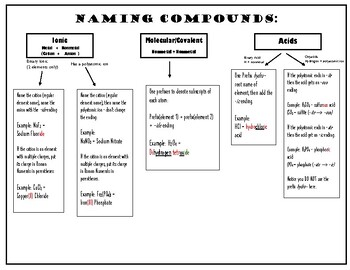
1. Understanding the Basics of Chemical Nomenclature

Chemical nomenclature involves a set of rules for naming compounds. Here are some basics:
- Binary Ionic Compounds: Consist of two elements where one is a metal and the other a non-metal. The metal’s name stays the same, while the non-metal’s name ends in “-ide.” E.g., NaCl (Sodium Chloride).
- Binary Covalent Compounds: Both elements are non-metals, and prefixes like “mono-,” “di-,” etc., indicate the number of atoms. E.g., CO (Carbon monoxide).
- Polyatomic Ions: These are groups of atoms carrying a charge. Names like “sulfate,” “nitrate,” etc., are used. E.g., Na₂SO₄ (Sodium sulfate).
⚗️ Note: The IUPAC (International Union of Pure and Applied Chemistry) provides the standard rules for chemical nomenclature globally.
2. Transition Metal Compounds

Naming compounds with transition metals involves:
- Using Roman numerals in parentheses to indicate the metal’s oxidation state. E.g., CuCl (Copper(I) chloride) and CuCl₂ (Copper(II) chloride).
- If the metal has a common name for its different states, this can be used. E.g., Ferrous (II) and Ferric (III) for iron.

3. Handling Acids

The naming of acids depends on:
- Hydrogen and an anion: Use “hydro-” prefix with the anion ending in “-ic” and add “acid” at the end. E.g., HCl (Hydrochloric acid).
- Polyatomic ions: For acids containing polyatomic ions, modify the ion’s name:
- ”-ate” becomes “-ic” and “acid.” E.g., HNO₃ (Nitric acid).
- ”-ite” becomes “-ous” and “acid.” E.g., HNO₂ (Nitrous acid).
4. Complex Ions and Coordination Compounds

The nomenclature for complex ions and coordination compounds involves:
- Naming ligands first, then the metal, followed by the oxidation state in Roman numerals in parentheses.
- For negatively charged complexes, the ending of the central metal changes to “-ate.”
- Examples:
- [Co(NH₃)₆]³⁺: Hexaamminecobalt(III) ion.
- [PtCl₄]²⁻: Tetrachloroplatinate(II) ion.

5. Organic Compounds

Naming organic compounds requires:
- Identifying the longest carbon chain, the root name.
- Using prefixes and suffixes to indicate substituents and functional groups:
- Alkanes end in “-ane,” alkenes in “-ene,” and alkynes in “-yne.”
- Alcohols use “-ol,” carboxylic acids “-oic acid,” and so on.
- Following IUPAC rules for correct naming, ensuring the name reflects the structure and functional groups accurately.
Key Points to Remember

- Always check for polyatomic ions or unique naming conventions.
- Pay attention to the oxidation state for transition metals.
- Understand the difference between ionic, covalent, and coordination compounds.
In wrapping up, mastering the art of naming chemical formulas is crucial for anyone involved in the chemical sciences. It not only ensures clear communication but also reflects an understanding of chemical bonding and properties. Whether in research, education, or industry, precise nomenclature helps avoid confusion, reduce risks, and promote accurate scientific discourse.
Why is it important to use Roman numerals in naming transition metal compounds?
+
Transition metals often have multiple oxidation states, which need to be clearly identified for accurate chemical formulas. Roman numerals indicate the oxidation state of the metal, distinguishing between compounds like Iron(II) oxide and Iron(III) oxide, which have different properties.
How do you know if a compound is an acid when naming it?

+
A compound is considered an acid if it releases hydrogen ions (H⁺) when dissolved in water. Look for hydrogen (H) bonded to nonmetals, particularly elements like Cl, S, N, or polyatomic ions like SO₄²⁻, NO₃⁻. The prefix “hydro-” and the suffix “-ic” or “-ous” acid help in naming acids.
What are the differences in naming between alkanes, alkenes, and alkynes?

+
Alkanes have only single bonds and end in “-ane”; alkenes contain at least one double bond, ending in “-ene”; alkynes have at least one triple bond, ending in “-yne.” The number of carbon atoms in the longest chain also dictates the prefix used (e.g., meth-, eth-, prop-).



