Moles to Particles: Atoms & Molecules Conversion Guide

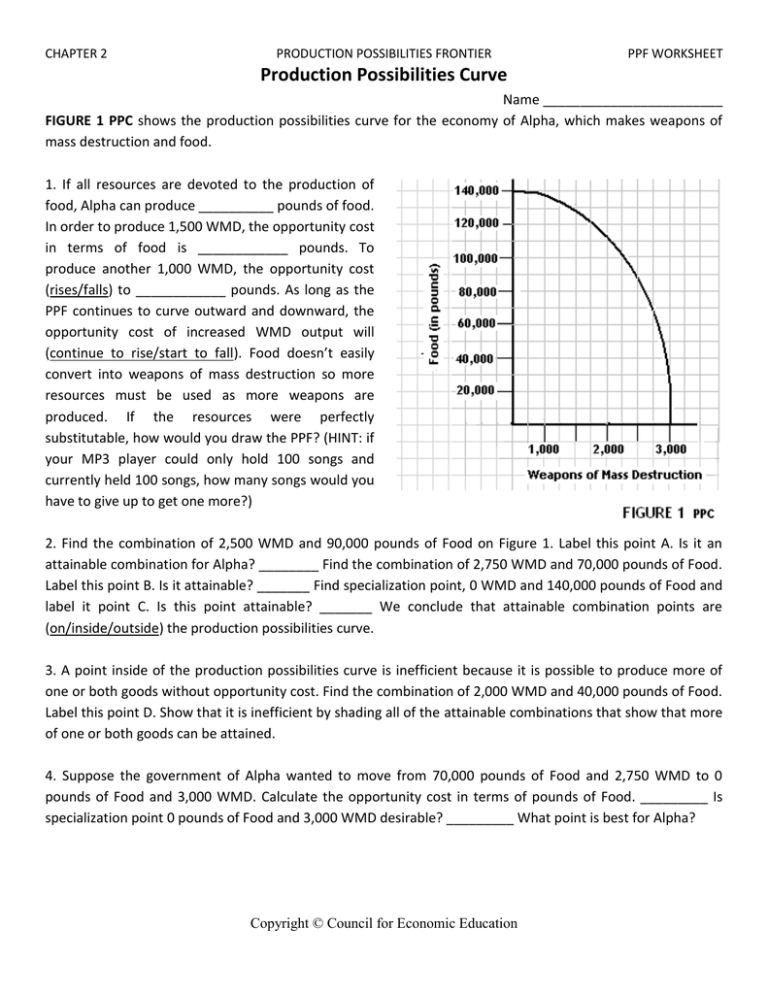
Understanding the Foundation: Moles and Avogadro's Constant

To master the art of converting moles to particles, one must first grasp the concept of moles and Avogadro’s number. A mole is a unit that chemists use to express amounts of substances, analogous to how “dozen” refers to twelve items. One mole is defined as exactly 6.022 x 1023 entities, which can be atoms, molecules, ions, or any other particle.
- A mole is denoted by the symbol "mol."
- This number, known as Avogadro's number, provides a way to count particles on a macroscopic scale.
Avogadro’s number (6.022 x 1023) is a fundamental constant of chemistry, named after Amedeo Avogadro. It links the microscopic world of atoms and molecules to the macroscopic world we observe, making it possible to perform calculations in a consistent and understandable manner.
🔍 Note: Avogadro's number has been precisely determined by experiments and is used internationally to convert between the number of particles and the amount of substance in moles.
Calculating Moles to Particles: Step-by-Step

Converting moles to the number of particles or vice versa requires a straightforward process:
- Identify the Conversion Factor: The conversion factor between moles and particles is Avogadro's number. Thus, 1 mol of substance contains Avogadro's number of particles.
- Set Up the Equation: Use the formula:
Number of Particles = Moles x Avogadro's number - Plug in the Values: Multiply the number of moles by Avogadro's number to find the number of particles.
For example, if you have 2 moles of a substance:
<table>
<tr><th>Quantity</th><th>Unit</th><th>Number of Particles</th></tr>
<tr><td>2 moles</td><td>mol</td><td>2 x 6.022 x 10<sup>23</sup></td></tr>
</table>
This results in approximately 1.204 x 1024 particles.
Atoms vs. Molecules: The Difference in Conversion

When converting moles, it’s crucial to differentiate between atoms and molecules:
- Atoms are the basic building blocks of matter and cannot be broken down further without losing their chemical identity.
- Molecules consist of two or more atoms bonded together, which can be of the same or different elements.
For conversions involving molecules, remember that:
<table>
<tr><th>Item</th><th>Units</th><th>Conversion</th></tr>
<tr><td>Moles of Molecules</td><td>mol</td><td>Number of Molecules = Moles x Avogadro's number</td></tr>
</table>
For example, 1 mole of water (H2O) has:
<table>
<tr><th>Item</th><th>Units</th><th>Number of Molecules</th></tr>
<tr><td>Water Molecules</td><td>mol</td><td>1 x 6.022 x 10<sup>23</sup></td></tr>
</table>
This corresponds to 6.022 x 1023 water molecules, each consisting of 2 hydrogen atoms and 1 oxygen atom.
Conversion Errors and Precision

When performing these conversions, consider the following:
- Rounding: Significant figures should be taken into account. When dealing with Avogadro's number, rounding errors can lead to significant inaccuracies.
- Unit Awareness: Moles are an extensive property, meaning they depend on the amount of substance present. Ensure that the units in the equation match.
📏 Note: Even small errors in calculations involving Avogadro's number can lead to large discrepancies due to the exponential nature of the number.
The Mole Concept in Complex Calculations

Mole conversions extend beyond simple mole-to-particle calculations:
- Compounds: Calculate the number of molecules for any compound, including those with complex formulas.
- Molarity: Use moles when calculating molarity (concentration in moles per liter of solution).
- Gas Laws: Ideal gas laws often incorporate moles to relate volume, temperature, and pressure.
The Implications of Molar Conversions

The ability to convert between moles and particles has significant implications in:
- Chemical Reactions: Balancing equations and stoichiometry require understanding mole ratios.
- Analytical Chemistry: Quantifying the amount of a substance in a sample.
- Material Science: Predicting and designing material properties based on their molecular or atomic makeup.
The journey of understanding and utilizing mole conversions opens doors to a deeper comprehension of the molecular world. By mastering this skill, you can move seamlessly between the tangible, macroscopic world of chemical labs and the microscopic universe of atoms and molecules.
Wrapping Up:
The conversion between moles and particles, facilitated by Avogadro’s number, is a cornerstone of chemical calculations. It connects the observable quantities we measure in the lab with the atomistic view of matter. This transformation from macroscopic to microscopic and back is not just a mathematical exercise; it’s a gateway to understanding chemical behavior, reactions, and material properties. Whether it’s for balancing reactions, determining empirical formulas, or quantifying substances, mole conversions are indispensable tools in the chemist’s arsenal. They allow us to peer into the molecular dance of atoms, creating a bridge between the invisible world of particles and the tangible results of chemical experiments.
What is Avogadro’s number used for?

+
Avogadro’s number is used to convert between the number of particles (atoms or molecules) and the amount of substance in moles, facilitating the quantification of substances in chemistry.
Can you convert moles to grams?

+
Yes, you can convert moles to grams by multiplying the number of moles by the molar mass of the substance. Molar mass is the mass of one mole of the substance in grams per mole (g/mol).
How does the mole concept affect gas laws?

+
The mole concept is integral to gas laws. The ideal gas law, PV=nRT, uses ‘n’ to represent the number of moles of gas, linking moles to pressure, volume, and temperature in gaseous systems.