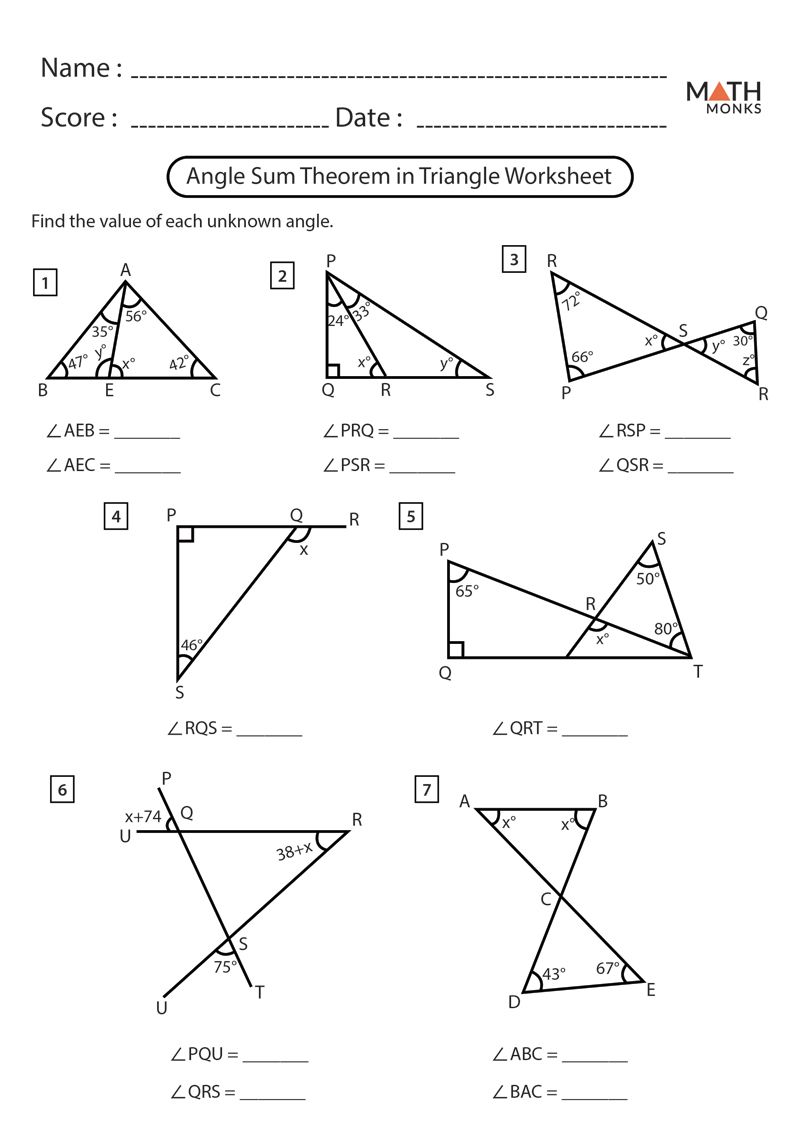
Master Mole to Mole Stoichiometry with Our Worksheet Answers

Understanding the fundamental principles of chemistry often involves grappling with intricate concepts such as stoichiometry. Stoichiometry allows chemists to calculate the quantities of reactants and products in a chemical reaction, ensuring that materials are used efficiently and safely. Mole to mole stoichiometry is particularly crucial because it enables us to translate between amounts of different substances in a reaction. This detailed guide will explore how to master mole to mole stoichiometry through practical examples, worksheets, and essential learning tips.
What is Mole to Mole Stoichiometry?

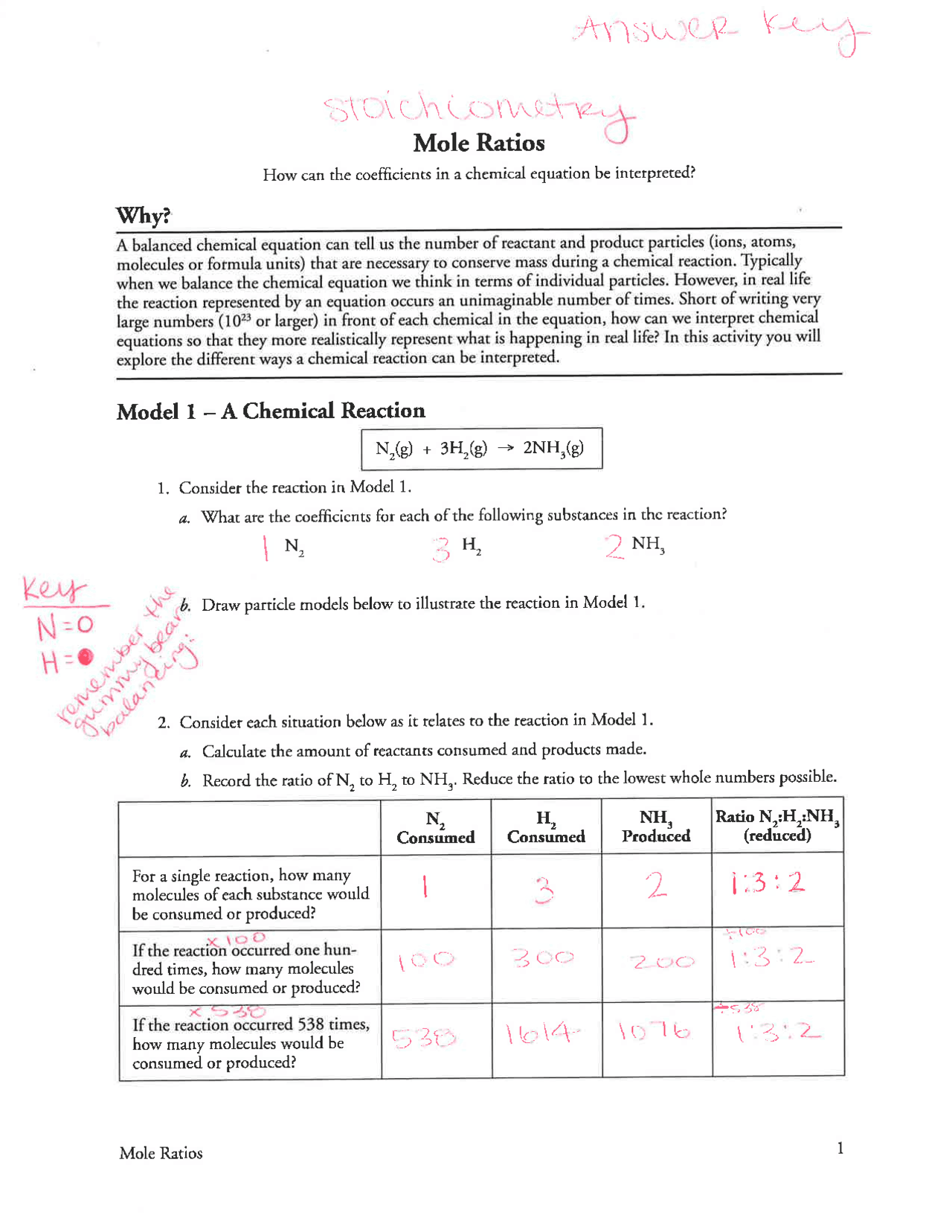
Mole to mole stoichiometry involves the calculation of the number of moles of one substance in a chemical equation relative to the moles of another. This relationship is derived from the coefficients found in balanced chemical equations. For example, consider the reaction: ```2H2 + O2 → 2H2O``` Here, we can see: - 2 moles of hydrogen react with 1 mole of oxygen to produce 2 moles of water.
💡 Note: The coefficients represent the molar ratio of the substances involved in the reaction.
Step-by-Step Guide to Solving Mole to Mole Problems

To master this concept, let’s delve into the steps:
Write and Balance the Chemical Equation: Start with a balanced equation to ensure the stoichiometric coefficients are correct.
Identify the Given and Unknown Moles: Determine what information is given (e.g., moles of one reactant or product) and what you need to find.
Set Up the Mole Ratio: Using the balanced equation, set up a ratio of the known substance to the unknown substance.
Perform the Calculation: Apply the mole ratio to solve for the unknown quantity:
Let’s say you want to find out how many moles of H₂O can be produced from 3 moles of H₂.
From the equation:
\frac{2 moles H₂O}{2 moles H₂} = \frac{x moles H₂O}{3 moles H₂}- Solving for x:
x = \frac{2}{2} * 3 = 3 moles H₂OCheck Your Units: Make sure your final answer is in moles.
- Note that these steps are not always linear; sometimes you'll need to adapt or repeat steps depending on the problem's complexity.
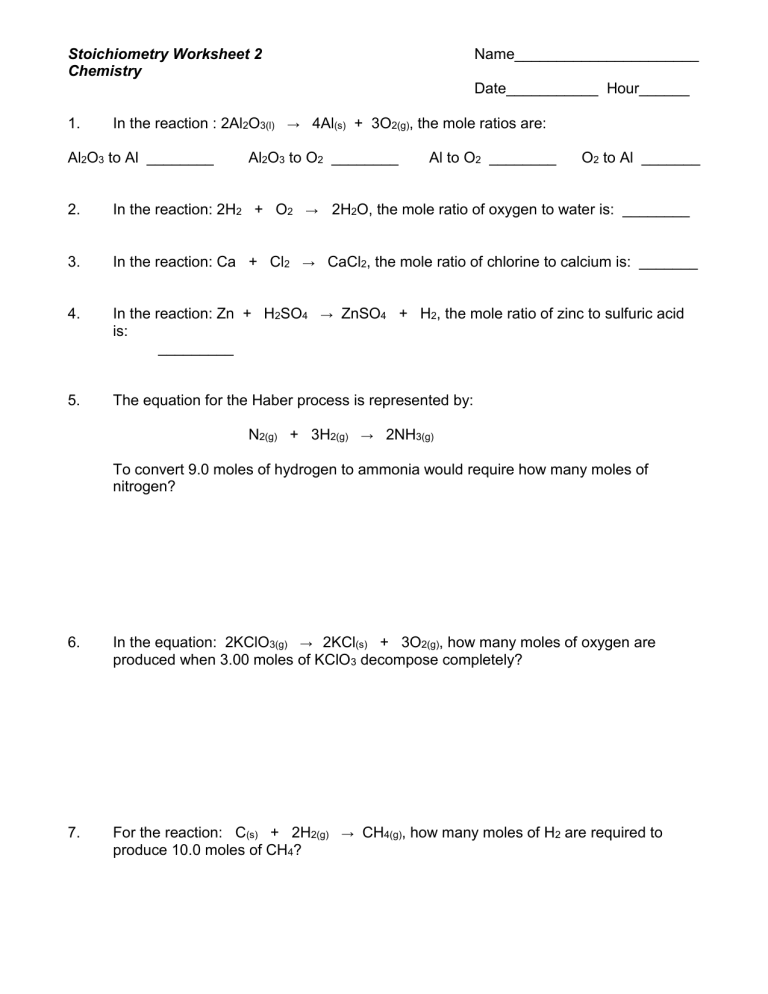
Worksheet Example

Let’s practice with a worksheet example:
| Question | Balanced Equation | Moles Given | Moles Sought |
|---|---|---|---|
| How many moles of N₂ are produced when 6 moles of NH₃ react? | 4NH₃ + 5O₂ → 4NO + 6H₂O | 6 moles of NH₃ | Moles of N₂? |
| Answer | 6 moles NH₃ × (1 mole N₂ / 4 moles NH₃) = 1.5 moles N₂ |

🧪 Note: Always ensure that the reaction is balanced before setting up your ratios.
Common Mistakes in Mole to Mole Stoichiometry

- Using unbalanced equations: Incorrect stoichiometry leads to incorrect calculations.
- Ignoring units: Moles are the only unit to use; never mix with grams or other units without conversion.
- Forgetting the significance of zero: Sometimes, zero moles can be the correct answer if there’s not enough reactant for the reaction to occur.
Tips to Master Mole to Mole Stoichiometry
- Practice: Engage with various worksheet exercises to reinforce your understanding.
- Concept Maps: Visualize the relationship between substances using concept maps or flowcharts.
- Review Mole Concepts: Make sure your foundational understanding of moles is solid.
To sum up, mastering mole to mole stoichiometry is about understanding how moles interact in chemical reactions. By following a systematic approach, practicing with various examples, and avoiding common pitfalls, you can become proficient in this essential chemistry skill. Whether you’re preparing for a test, working in a lab, or simply exploring chemistry for personal interest, the ability to solve these problems quickly and accurately can greatly enhance your understanding and efficiency in the subject.
Why is balancing chemical equations important in stoichiometry?
+
Balancing ensures that the law of conservation of mass is adhered to; it provides the correct molar ratios needed for stoichiometric calculations.
Can you convert between grams and moles directly in stoichiometry?

+
Yes, but first, you need to convert grams to moles using the molar mass of the substance before performing mole to mole calculations.
What if the reaction yields are not 100%?

+
Adjust your calculations by considering the percent yield; the actual yield will be less than the theoretical yield due to reaction inefficiencies or side reactions.
How can I ensure accuracy in my stoichiometric calculations?

+
Accuracy comes from practice, understanding the reaction, and double-checking your work. Use significant figures, balance the equation, and verify your units throughout the calculation.