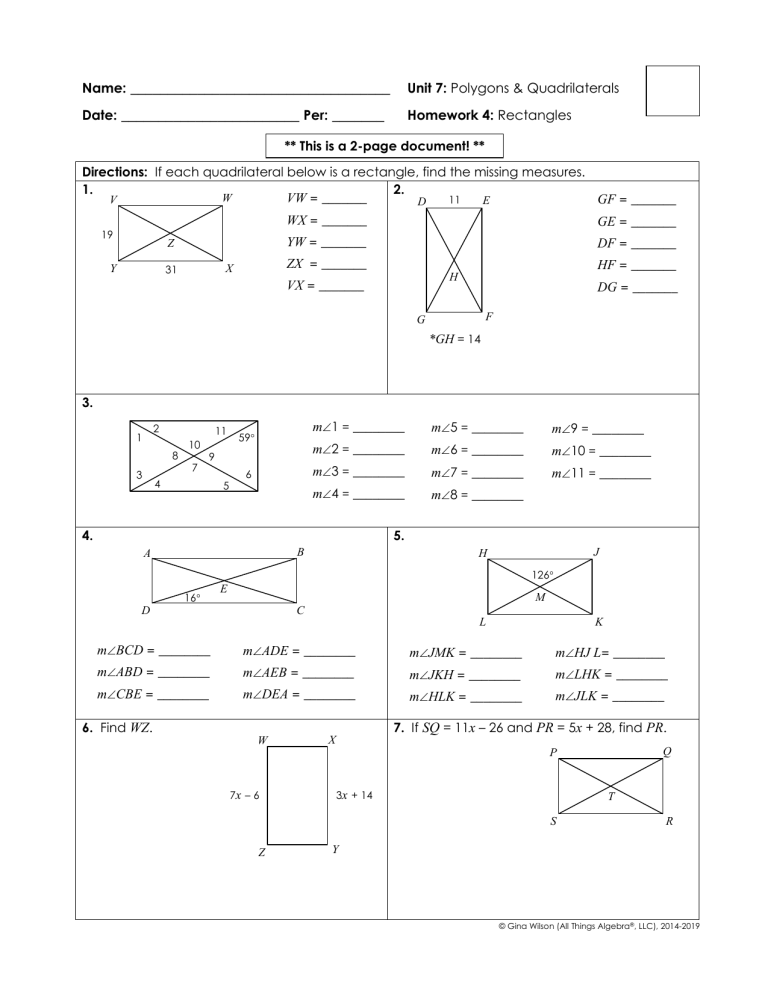
Mastering Mole to Mole Stoichiometry: Essential Practice Worksheet

Chemistry can sometimes feel like a labyrinth with intricate paths that lead you to the heart of understanding how substances react and change. Among these paths, mole to mole stoichiometry is one of the most fundamental yet challenging concepts for students to grasp. This blog post will guide you through the essential steps of mastering mole to mole stoichiometry using a practice worksheet, ensuring you understand this critical concept deeply.
Why Is Stoichiometry Important?

Stoichiometry is the backbone of chemical reactions, providing insights into the quantities of reactants and products in a reaction. It's not just about balancing equations but also about understanding:
- Reaction efficiency
- Production yield
- Resource allocation
Understanding Mole to Mole Conversions

Before diving into the practice worksheet, let's understand the basics:
- Moles: A mole is a unit used in chemistry to express amounts of a substance, representing Avogadro's number of entities (6.022 x 10^23).
- Stoichiometry: This term refers to the quantitative relationship between reactants and products in a chemical reaction.
The concept of mole to mole stoichiometry is crucial as it allows you to convert between different substances in a balanced chemical equation based on their molar ratios.
Steps to Solve Mole to Mole Stoichiometry Problems

Here are the steps to tackle these types of problems:
- Balance the chemical equation: Ensure the equation is balanced so that atoms of each element are the same on both sides.
- Identify the known and unknown: Determine which substances you know the amount for, and which you need to find.
- Set up the molar ratio: Use the coefficients from the balanced equation to set up the conversion factor.
- Calculate the unknown moles: Multiply the known amount by the molar ratio to find the moles of the unknown substance.
Let's apply this with an example:
Example Problem:
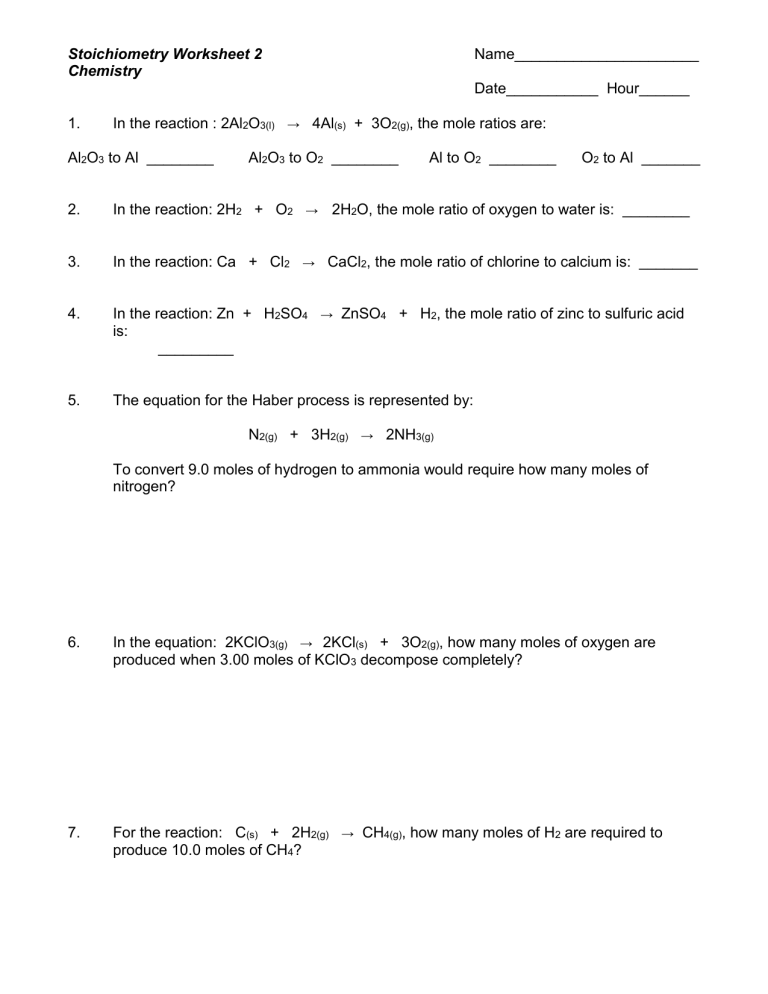
How many moles of O2 are required to burn 4.5 moles of methane (CH4) according to the following equation:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
🔬 Note: The coefficients in the balanced equation directly correspond to the moles of each reactant and product involved in the reaction.
Here's how we solve it:
- The equation is already balanced.
- We know the moles of CH4 (4.5 moles), and we need to find the moles of O2.
- The molar ratio from CH4 to O2 is 1:2 (one mole of CH4 reacts with two moles of O2).
- Calculation: 4.5 moles CH4 × (2 moles O2 / 1 mole CH4) = 9 moles of O2.
Practice Worksheet: Mole to Mole Stoichiometry

| Problem | Reaction | Given Moles | Find Moles | Answer |
|---|---|---|---|---|
| 1 | 2H2 + O2 → 2H2O | 3 moles of H2 | Moles of O2 | 1.5 moles of O2 |
| 2 | N2 + 3H2 → 2NH3 | 2.5 moles of N2 | Moles of NH3 | 5 moles of NH3 |
| 3 | CaCO3 → CaO + CO2 | 1 mole of CaCO3 | Moles of CO2 | 1 mole of CO2 |

⚠️ Note: Always check your calculations twice to ensure accuracy.
In mastering mole to mole stoichiometry, repetition through practice problems is key. By working through the above worksheet, you're not just solving equations; you're also building your intuition for the nature of chemical reactions.
The above steps and practice problems should guide you towards a stronger understanding of how to approach mole to mole stoichiometry. Remember:
- Balancing the equation is fundamental.
- Mole ratios from the balanced equation dictate the conversion factors.
- Precision in calculations prevents errors.
Why do I need to balance the equation in stoichiometry?

+
Balancing the equation ensures that the law of conservation of mass is observed in the chemical reaction, which is critical for accurate stoichiometric calculations.
What if the molar ratios aren’t whole numbers?

+
If the ratios are not whole numbers, you should simplify them to whole numbers for easier conversion, ensuring that the ratios still accurately represent the reaction.
Can stoichiometry be applied to all chemical reactions?

+
Yes, stoichiometry can be applied to all balanced chemical reactions to determine the quantitative relationship between reactants and products.