Master Mole to Mole Conversions: Ultimate Guide and Worksheet
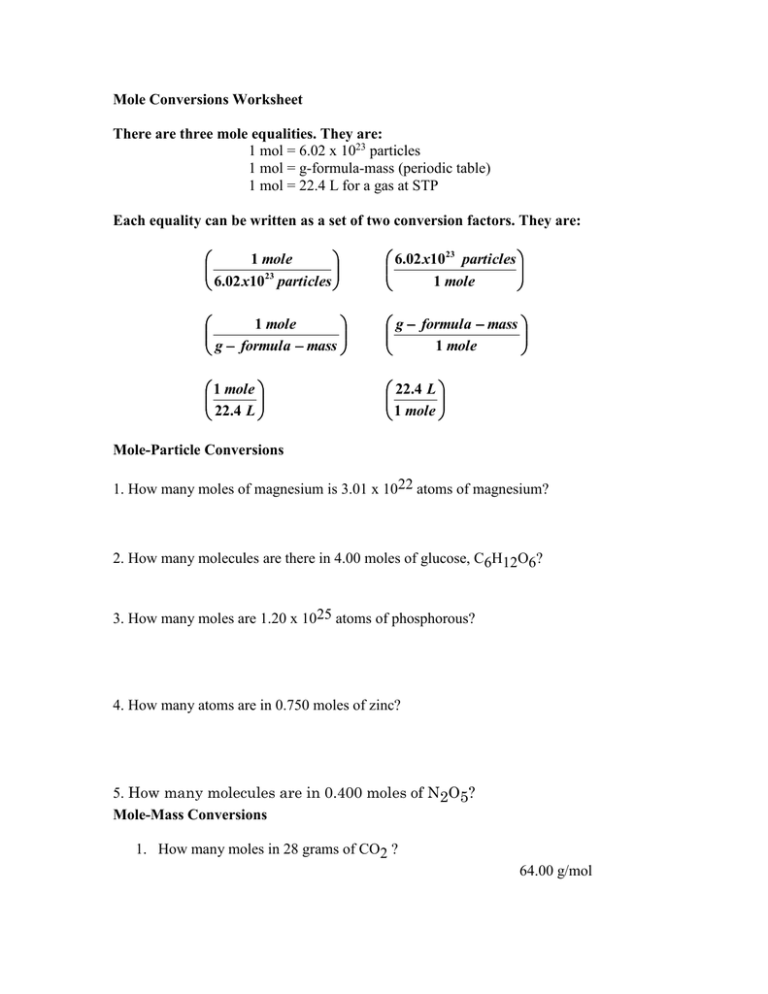
Mastering mole to mole conversions is fundamental for students delving into chemistry, particularly when dealing with chemical reactions, stoichiometry, and quantitative analysis. In this comprehensive guide, we'll unravel the intricacies of mole to mole calculations, providing you with a clear understanding and practical tools to master this essential chemistry concept.
Why are Mole to Mole Conversions Important?

Before diving into the how, let’s discuss the why. Mole to mole conversions are crucial because they allow us to understand:
- The amount of reactants and products in a chemical reaction
- How balanced chemical equations relate to actual quantities in the lab
- The concept of limiting reactants and excess reagents
- Stoichiometric relationships in chemical reactions
Without this understanding, it would be nearly impossible to predict or control chemical reactions, a task fundamental to industrial applications, pharmaceutical development, environmental analysis, and more.
The Basics of Mole Conversion

Here are the key elements you need to grasp before moving into conversions:
- Avogadro’s Number: One mole is equal to approximately 6.022 x 1023 particles, whether atoms, ions, or molecules.
- Molar Mass: The mass of one mole of any substance expressed in grams, which can be found using the periodic table.
- Chemical Equations: A balanced chemical equation provides the molar ratios of reactants to products, allowing mole to mole conversions.
Step-by-Step Guide to Mole to Mole Conversions

To perform mole to mole conversions, follow these steps:
- Identify the balanced chemical equation: This equation will show you the mole ratio between substances.
- Set up a ratio using the coefficients from the balanced equation: For example, if the equation is 2H2 + O2 → 2H2O, the ratio of H2 to O2 is 2:1.
- Convert the given moles into the moles of the substance you need: Use the ratio as a conversion factor. If you need to convert moles of O2 to moles of H2O, multiply by the ratio (2⁄1).
⚠️ Note: Always ensure your equation is balanced before converting to avoid inaccuracies.
Example: Mole to Mole Conversion Worksheet

Let’s apply the steps with an example:
Problem:

N₂ + 3H₂ → 2NH₃
If you start with 1.5 moles of H₂, how many moles of NH₃ can you produce?
Solution:
- Identify the balanced equation.
- Set up the ratio: From the equation, the ratio of H₂ to NH₃ is 3:2.
- Perform the conversion:
- 1.5 moles H₂ * (2 moles NH₃ / 3 moles H₂) = 1 mole NH₃
| Reactant | Moles Given | Moles Calculated |
|---|---|---|
| N₂ | Not given | Not Calculated |
| H₂ | 1.5 | - |
| NH₃ | - | 1.0 |

✅ Note: Always double-check your conversion factors and ratios for accuracy.
Practical Applications of Mole to Mole Conversions

Mole to mole conversions are not just academic exercises; they have real-world applications:
- Pharmaceutical Production: Ensuring correct proportions of chemicals to form the desired compound.
- Environmental Monitoring: Calculating pollutant emissions in terms of moles to understand environmental impact.
- Metallurgy: Controlling the amount of reactants in smelting processes.
These conversions help in scaling up from small lab experiments to industrial levels while maintaining reaction efficiency.
Final Thoughts on Mole to Mole Conversions

The ability to perform mole to mole conversions opens up the world of chemistry. It’s a foundational skill that allows you to predict and quantify how substances interact in reactions, facilitating both theoretical understanding and practical application in various scientific fields. Mastery of this concept not only boosts your competence in stoichiometry but also prepares you for more advanced chemical calculations and experiments.
What is Avogadro’s number?

+
Avogadro’s number is approximately 6.022 x 1023, and it represents the number of particles in one mole of any substance. It’s named after Amedeo Avogadro, who proposed the theory that equal volumes of gases at the same temperature and pressure contain an equal number of molecules.
Why do I need to balance a chemical equation before converting moles?

+
Balancing a chemical equation ensures that the law of conservation of mass is followed, meaning the number of atoms for each element is the same on both sides of the equation. This balance provides the correct molar ratios necessary for accurate mole to mole conversions.
Can you convert from grams to moles and then do a mole to mole conversion?

+
Absolutely. You first convert the mass of a substance to moles using its molar mass, then use the balanced equation to convert those moles into moles of another substance. For example, if you know the mass of oxygen in grams, convert it to moles of oxygen, then use the mole ratio from a balanced equation to find moles of a product.



