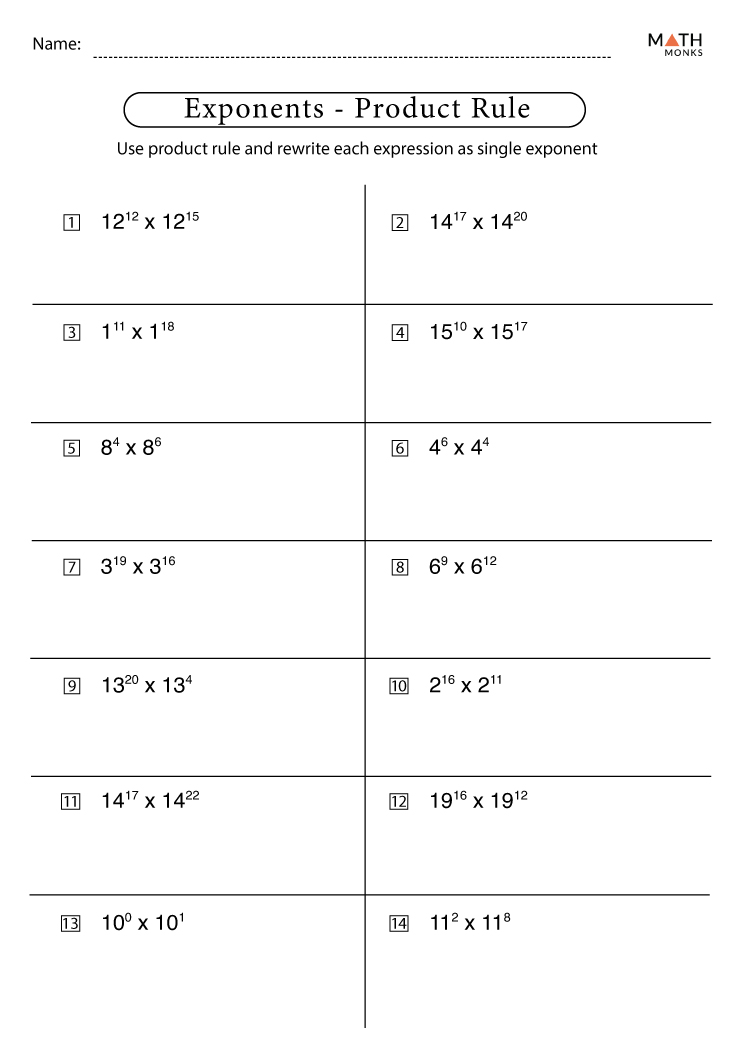
5 Ways to Master Molarity Problems: Answer Key Included

In the intricate world of chemistry, understanding molarity is essential for students who want to excel in their labs and exams. Molarity, which is represented by the symbol M, measures the concentration of a solute in a solution, specifically, the number of moles of solute per liter of solution. Mastering molarity problems not only helps in accurately preparing solutions but also in understanding reaction stoichiometry, dilution techniques, and much more. Here are five effective ways to conquer these challenges, along with an answer key to guide your practice.
1. Understand the Basics of Molarity


The foundation of molarity calculations lies in the formula:
Molarity (M) = moles of solute / volume of solution (in liters)- Moles of solute: The amount of solute present in moles.
- Volume of solution: The total volume of the solution in liters.
Understanding this relationship is key because it allows you to calculate:
- How much solute is required for a given concentration and volume.
- The volume of solution needed for a specific amount of solute.
2. Use Dimensional Analysis

Dimensional analysis, or the factor-label method, is a powerful tool in solving molarity problems:
- Convert the units systematically to cancel out unwanted units.
- Link known quantities to the unknown with conversion factors (e.g., molar mass, volume, molarity).
3. Practice Solving Problems

The adage “practice makes perfect” holds true with molarity calculations:
- Solve various types of problems: dilution, finding molarity from mass, preparing solutions, etc.
- Use real-life scenarios or lab protocols to apply the concepts practically.
- Check your calculations with the following answer key:
| Problem | Solution |
|---|---|
| Calculate the molarity of a solution containing 0.50 mol of NaCl in 250 mL of solution. | M = (0.50 mol) / (0.25 L) = 2.0 M |
| Determine the moles of NaOH in 150 mL of a 0.10 M solution. | moles = (0.10 M) * (0.150 L) = 0.015 mol |
| How much KCl (in grams) is required to prepare 500 mL of a 1.5 M solution? | moles needed = (1.5 M) * (0.5 L) = 0.75 mol mass of KCl = (0.75 mol) * (74.55 g/mol) = 55.91 g |

4. Relate Molarity to Volume and Mass

Recognize that molarity is not just about the solution; it connects volume, mass, and the chemical properties of substances:
- Use molar mass to convert between grams and moles.
- Understand how changes in volume affect molarity when diluting solutions.
⚗️ Note: When you dilute a solution, remember that the moles of solute remain constant, but the molarity decreases as the volume increases.
5. Master Dilution Calculations

Dilution is a frequent application of molarity in the lab:
M1V1 = M2V2- M1: Initial molarity
- V1: Initial volume
- M2: Final molarity
- V2: Final volume
Understanding dilution allows you to:
- Calculate the required volume to reach a desired concentration.
- Accurately prepare serial dilutions.
In mastering molarity problems, it's not just about knowing the formulas but understanding the principles of chemistry they represent. Here's what you need to remember:
By focusing on these key methods, students can effectively master molarity problems:
- Develop a deep understanding of the molarity equation.
- Utilize dimensional analysis for efficient problem-solving.
- Practice regularly with diverse problems.
- Relate molarity to the practical aspects of chemistry.
- Master the art of dilution for experimental work.
Why is molarity important in chemistry?

+
Molarity is essential because it allows chemists to quantify the amount of a solute in a solution, which is critical for experiments, reactions, and lab procedures. It helps in determining reaction rates, stoichiometric relationships, and solution preparation.
What are common mistakes in molarity calculations?

+
Common errors include not converting units correctly (especially from milliliters to liters), misapplying conversion factors, or forgetting that molarity depends on both the number of moles and the volume of the solution.
How does molarity change with dilution?

+
When a solution is diluted, the molarity decreases because the number of moles of solute remains constant while the volume of the solution increases, spreading out the solute over a larger volume.
Can you perform dilution without changing molarity?

+
Yes, if you add more solvent while keeping the moles of solute constant, you are effectively diluting the solution, but the molarity would decrease proportionally to the increase in volume.
How do you prepare a solution of a specific molarity?

+
To prepare a solution with a specific molarity, calculate the moles of solute needed for the desired volume, then weigh out that amount of solute, dissolve it in some solvent, and finally dilute to the total volume required.