Master the Art of Dilutions with Our Worksheet
Mastering the art of dilutions is a crucial skill in various scientific fields, especially chemistry, biology, and medicine. Understanding how to prepare and work with solutions of different concentrations can streamline your experiments and ensure accurate results. In this comprehensive guide, we'll explore the intricacies of dilutions, including the terminology, methods, and practical applications. Whether you're a student, a budding scientist, or a seasoned researcher, this blog post will provide valuable insights and tools to master dilutions effectively.
Understanding the Basics of Dilutions

Dilution is the process of reducing the concentration of a solute in a solution by adding more solvent. Here are some fundamental concepts to grasp:
- Concentration: The amount of solute dissolved in a given amount of solvent or solution. Common units include molarity (M), molar (M), and grams per liter (g/L).
- Solute: The substance being dissolved in the solvent.
- Solvent: The liquid used to dissolve the solute, typically water in lab settings.
- Stock Solution: A concentrated solution from which diluted solutions are prepared.
- Diluent: A solvent used to dilute the stock solution.
The Dilution Formula
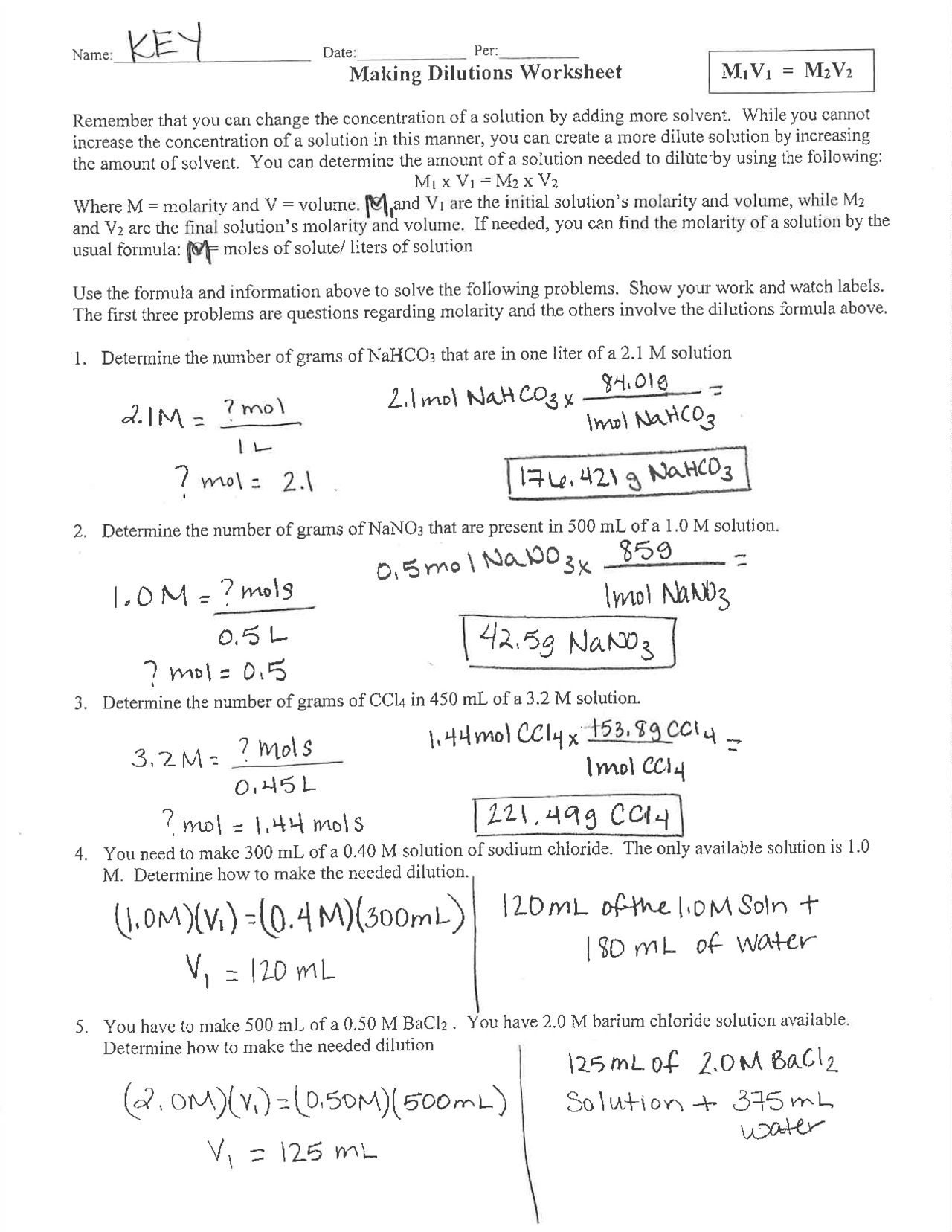
The primary formula for calculating dilutions is:
- C1V1 = C2V2
Where:
- C1 = Concentration of the stock solution
- V1 = Volume of the stock solution to be taken
- C2 = Desired concentration of the diluted solution
- V2 = Total volume of the final diluted solution
To understand this formula better, let's use an example:
Example:
Suppose you have a 10 M stock solution of sodium chloride (NaCl) and you need to prepare 100 mL of a 1 M NaCl solution.
- C1 = 10 M
- V1 = ?
- C2 = 1 M
- V2 = 100 mL
Using the formula:
- 10V1 = 1*100
- V1 = 10 mL
🔬 Note: Always double-check your calculations to avoid errors in solution preparation.
Tools for Dilution

To perform dilutions accurately, several tools are indispensable:
| Tool | Purpose |
|---|---|
| Volumetric Flask | Used for precise volume measurements and mixing solutions to a specified volume. |
| Pipettes | For accurately measuring and transferring small volumes of liquid. |
| Graduated Cylinders | Measuring the volume of larger amounts of liquid. |
| Micropipettes | Enables precise dispensing of microliter volumes. |
| Beakers | For rough volume measurements when exact volume is not critical. |

Steps for Diluting a Solution

Here’s how you can approach diluting a stock solution:
- Determine the Volume of Stock Solution Needed: Use the dilution formula to find the volume of the stock solution required.
- Select the Proper Container: Depending on the volume of your diluted solution, choose between a volumetric flask or a graduated cylinder.
- Transfer the Stock Solution: Using a pipette or a micropipette, transfer the calculated volume of the stock solution into your container.
- Add Solvent: Fill the container with the solvent (e.g., water) up to the mark for your desired final volume.
- Mix Well: Mix the solution thoroughly to ensure even distribution of the solute.
💧 Note: When using water as the solvent, distilled or deionized water is preferred to avoid contamination.
Practical Tips for Dilutions
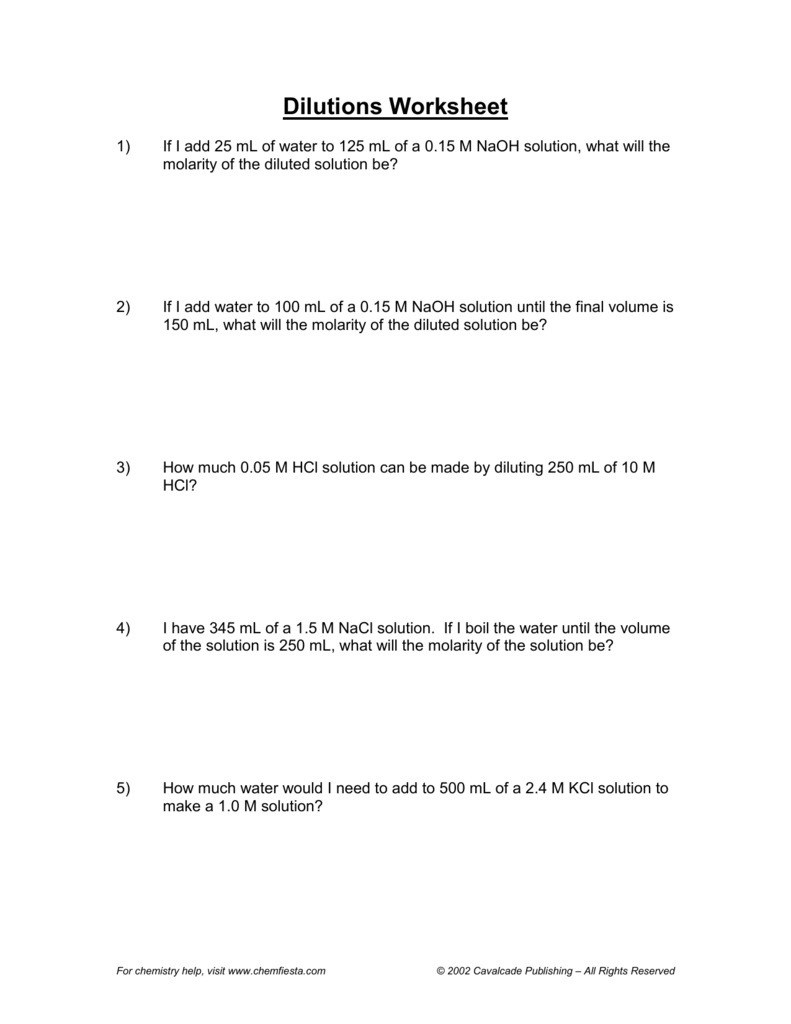
Here are some practical tips to ensure you master the art of dilutions:
- Accuracy: Use calibrated equipment for measurements to minimize errors.
- Stir to Homogeneity: After adding the solvent, stir or shake gently to ensure the solution is homogeneous.
- Check for Sediment: If a precipitate forms, you might need to gently warm the solution or use a different solvent.
- Serial Dilutions: For large dilution factors, perform serial dilutions to reduce the error margin.
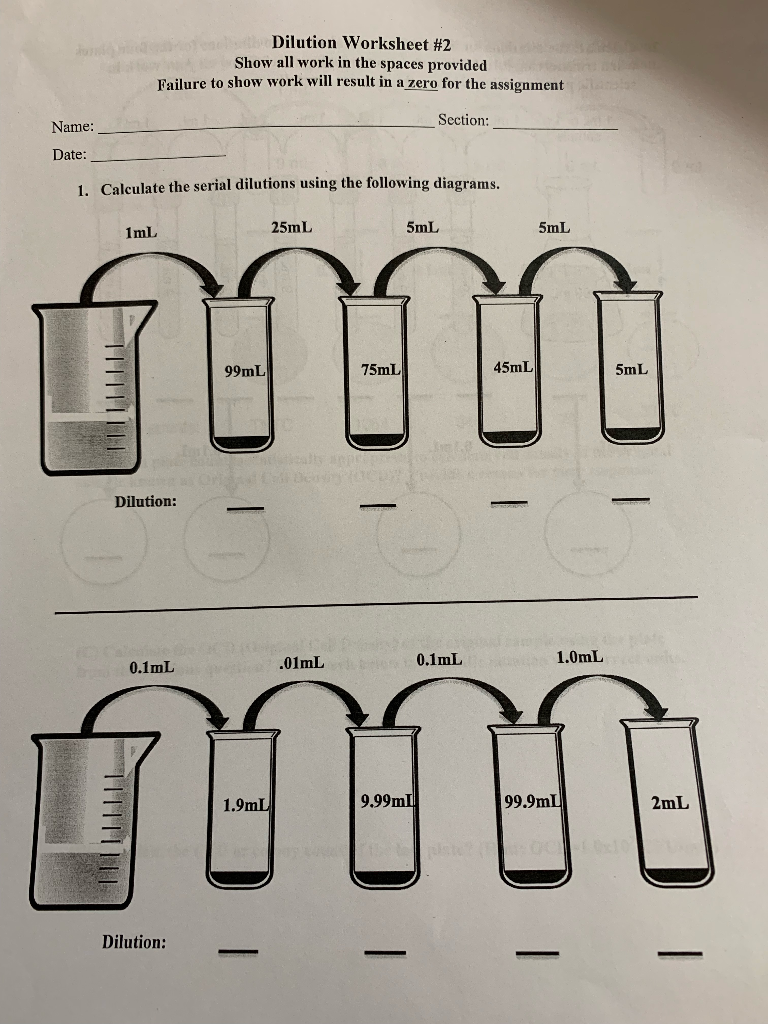
Serial Dilutions

Serial dilution involves making a series of successive dilutions, often used when dealing with very concentrated solutions or to achieve large dilution factors:
- Take a known volume of the stock solution into a container.
- Add solvent to reach the desired volume, creating the first dilution.
- From this diluted solution, take another known volume, and dilute it again in a new container.
- Repeat as many times as necessary.
Applications of Dilutions

Dilutions are critical in various scientific applications:
- Pharmacology: To prepare drug concentrations for testing or administration.
- Biochemistry: For enzyme and antibody assays.
- Environmental Science: In analyzing water quality or soil samples.
- Microbiology: To count bacterial populations or isolate single colonies.
- Chemistry: Standard solutions for titration or spectrophotometry.
The skill of diluting solutions correctly is fundamental to the accuracy and reliability of your scientific experiments and processes. With the knowledge of dilution principles, tools, and techniques, you can enhance the precision of your work in the laboratory.
In this guide, we've covered how to understand and apply dilutions in various scientific contexts, ensuring you can prepare solutions of specific concentrations with confidence. Dilution isn't just about mixing liquids; it's about achieving the desired effect in your experiments with precision and accuracy. Whether you're adjusting the strength of a reagent or preparing a solution for an assay, your ability to perform dilutions will significantly influence your results.
Why is dilution important in science?

+
Dilution is crucial for accurately measuring concentrations, reducing toxicity or osmolarity, and preparing standards for experimental protocols.
What are the common mistakes in dilution?

+
Common errors include miscalculation of volumes, using inaccurate measuring tools, not mixing solutions well, and not accounting for temperature effects on volume.
How can I check if my dilution is accurate?

+
Conduct a spectrophotometric analysis or use conductivity measurements if applicable. Alternatively, you can prepare known standards and compare with known solutions using a calibration curve.