Master Limiting Reactant Calculations with Worksheet #2
Chemistry, as fascinating as it is, often comes with a plethora of calculations that can be a stumbling block for many students. Among these, limiting reactant calculations stand out as a crucial part of stoichiometry where understanding how to determine which reactant runs out first, thus limiting the amount of product that can be formed, is essential. In this detailed exploration, we delve into worksheet #2 on limiting reactant calculations, providing step-by-step guidance to master this challenging topic.
Understanding Limiting Reactants
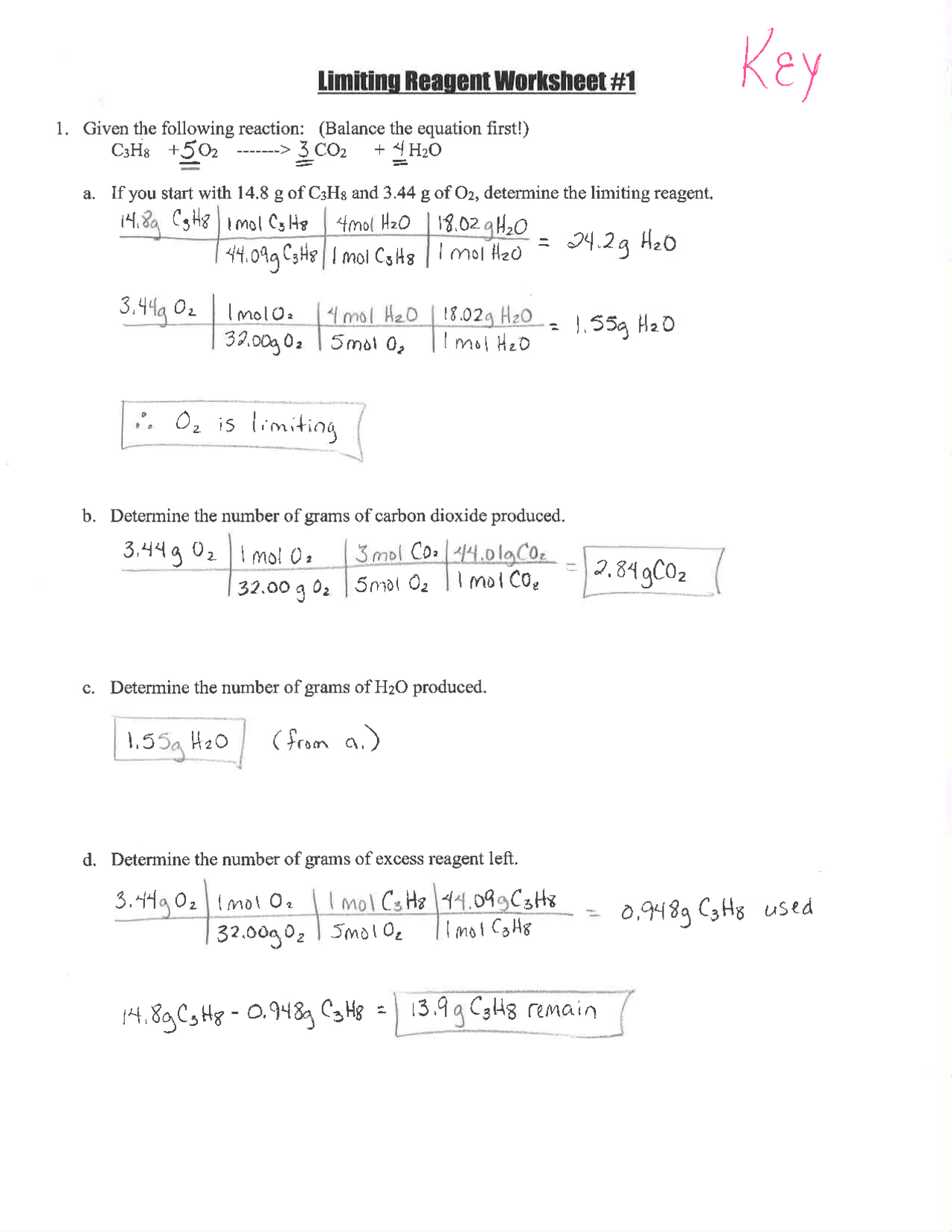
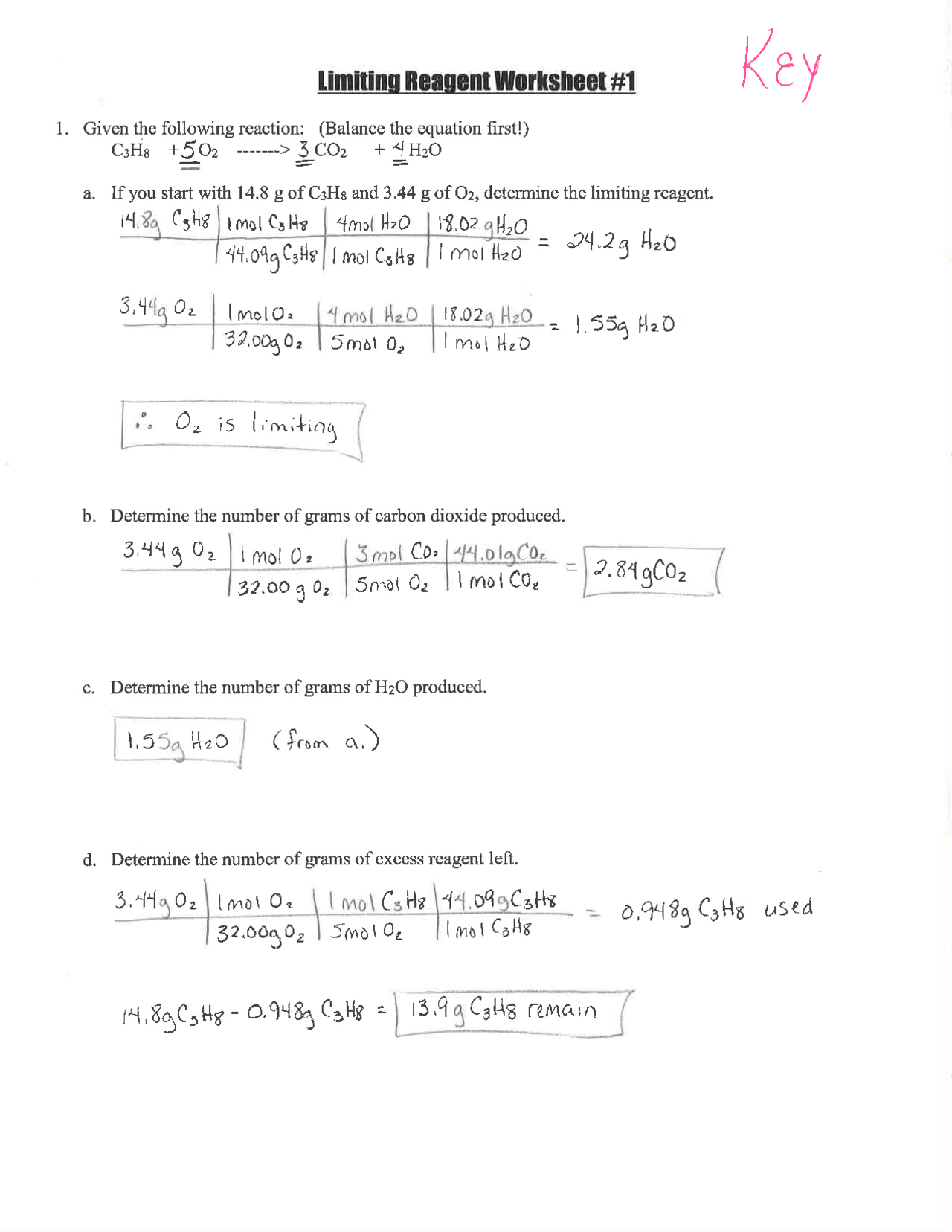
Before we dive into the worksheet, let’s cement the concept:
- Limiting Reactant: The reactant that is completely consumed in a chemical reaction, thereby limiting the formation of products.
- Excess Reactant: The reactant that is not fully consumed and remains after the reaction stops.
Worksheet #2: An Overview

The worksheet #2 provided typically involves several scenarios where reactants are mixed together in various molar ratios. Here’s how to approach the problems:
- Calculate Moles: Determine the moles of each reactant.
- Identify Limiting Reactant: Use stoichiometry to find the limiting reactant.
- Calculate Product Yield: Compute the theoretical yield of products based on the limiting reactant.
- Determine Excess: Identify how much of the excess reactant remains unreacted.
Step-by-Step Calculation for Limiting Reactant

Here’s a detailed look at how to solve these problems:
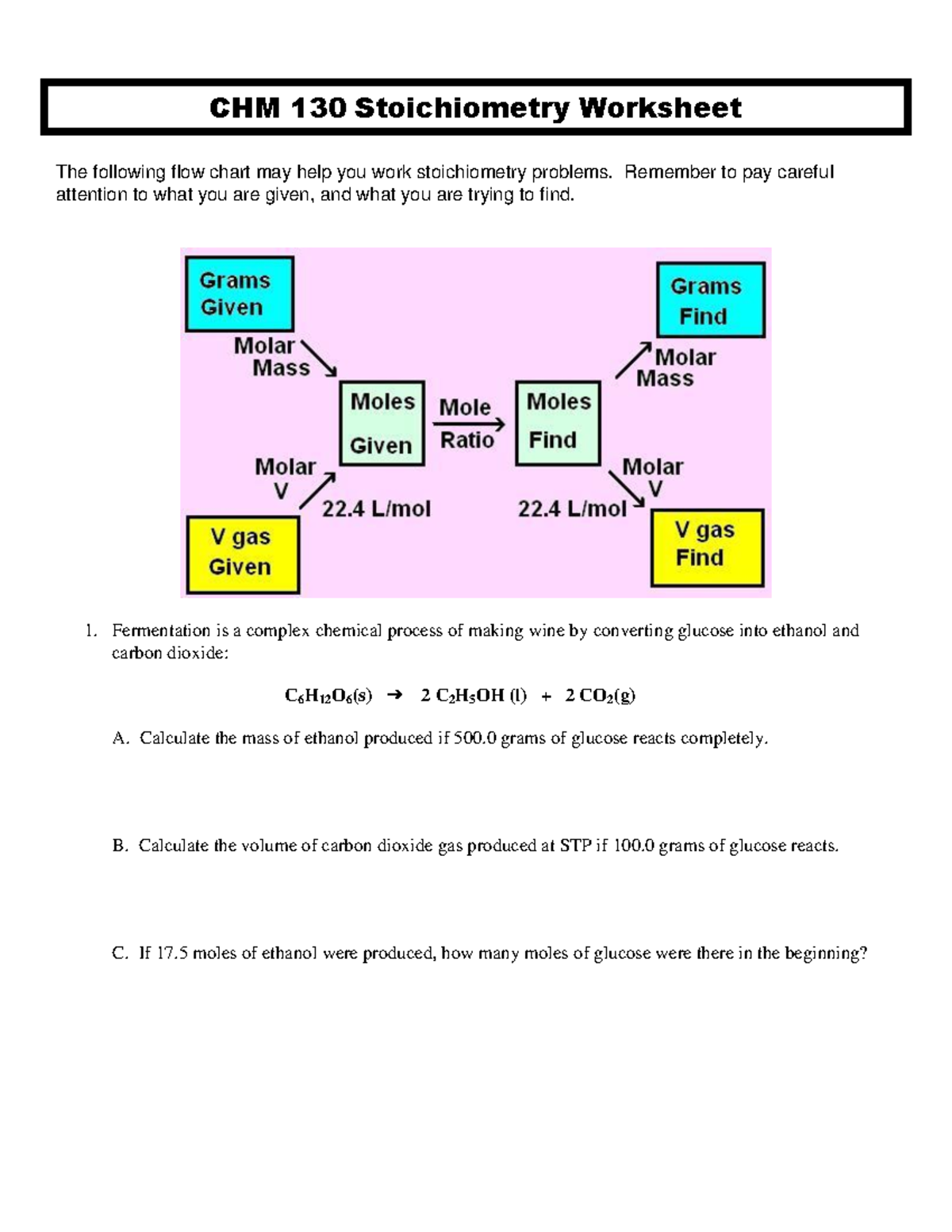
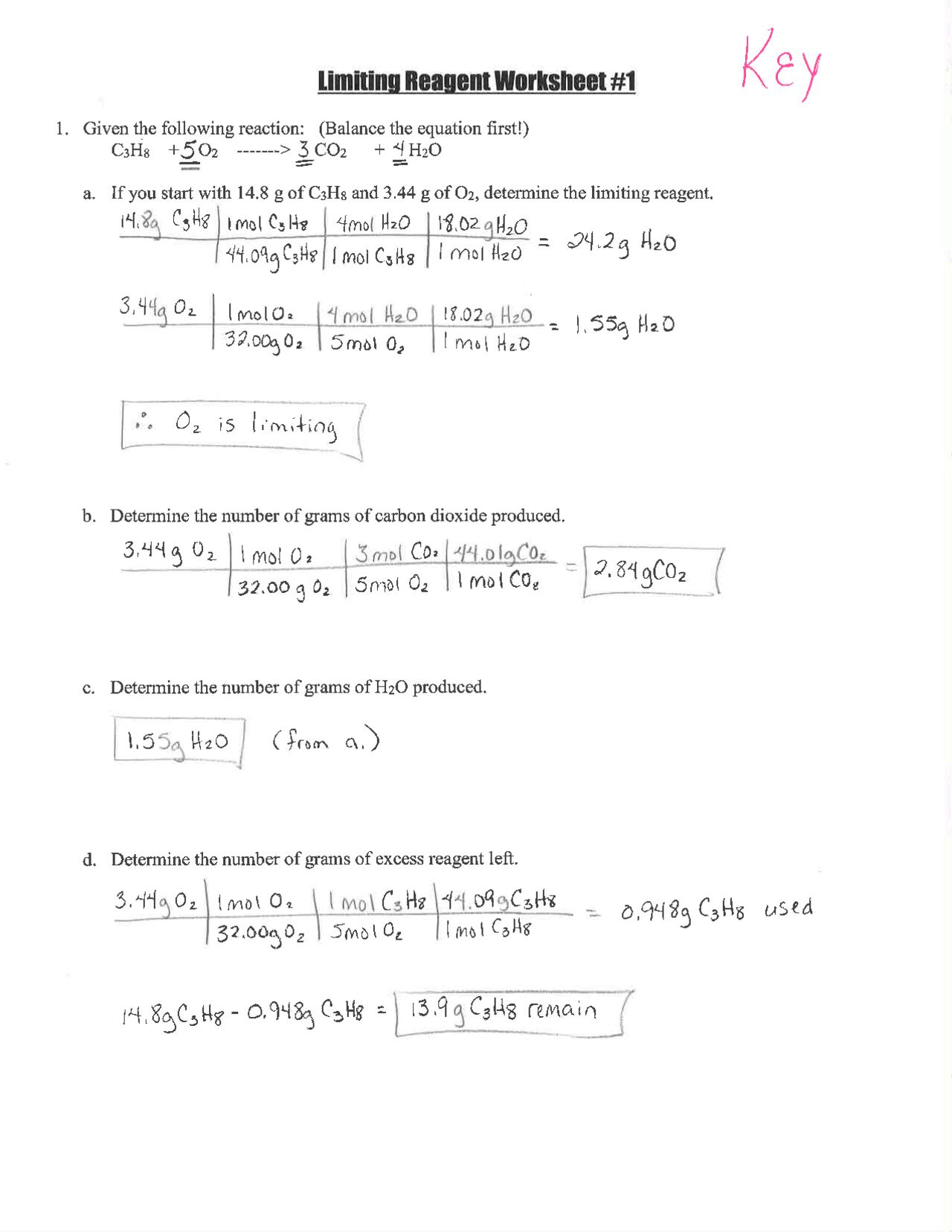
- Balance the Chemical Equation: Ensure the chemical equation is balanced before starting calculations.
- Determine Moles: Use the mass or concentration provided to calculate moles using the formula moles = mass/molar mass or moles = concentration x volume for solutions.
- Calculate the Mole Ratio: Based on the coefficients from the balanced equation, determine how much product can be formed from each reactant.
- Identify Limiting Reactant: Compare the results; the reactant that produces the least amount of product is the limiting reactant.
- Compute Product Yield: Use the moles of the limiting reactant to calculate the moles of product, then convert to mass if needed.
- Determine Excess Reactant: Subtract the moles of the reactant that reacted from the initial amount to find the excess.
⚠️ Note: Always double-check your mole ratio calculation from the balanced equation. Errors here lead to incorrect identification of the limiting reactant.
| Step | Description |
|---|---|
| 1 | Balance the equation |
| 2 | Calculate moles of reactants |
| 3 | Determine mole ratio |
| 4 | Identify the limiting reactant |
| 5 | Calculate theoretical yield |
| 6 | Determine excess reactant |

Practical Applications of Limiting Reactant Calculations

Limiting reactant calculations are not just academic exercises; they have real-world implications:
- Industrial Chemistry: Determining the cost-effectiveness and efficiency of production.
- Environmental Chemistry: For understanding pollutant formation and remediation.
- Food Science: In cooking and food processing, where ingredients must be accurately measured to avoid waste.
As we reach the end of our journey through worksheet #2, it's clear that mastering limiting reactant calculations is pivotal for any student aiming to excel in chemistry. By understanding and practicing these steps, students can predict reaction outcomes, manage reactants efficiently, and apply this knowledge to real-world scenarios. The logical progression from equation balancing to product yield calculation equips learners with a systematic approach to problem-solving that benefits not only in academics but in practical chemistry as well.
What if I get different answers for limiting reactant?
+
Double-check your calculations, particularly the mole ratios and the initial moles of reactants. Miscalculations often stem from using incorrect molar masses or forgetting to convert between grams and moles properly.
Can the limiting reactant change?

+
Yes, the limiting reactant can change depending on the amounts of reactants used. If you alter the initial quantities, you might find a different reactant limiting the reaction.
How do I know when I’ve found the limiting reactant?

+
Once you’ve calculated the moles of each product using the reactants, the reactant that results in the lowest yield of the product is the limiting reactant.