Limiting Reactant Problems: Step-by-Step Answers Guide

The Fundamentals of Limiting Reactants

When engaging with chemical reactions, particularly in stoichiometry, one will often come across the concept of a limiting reactant (or limiting reagent). This is the reactant that is completely consumed first in a reaction, halting further progression because there are no more molecules of that substance to react. Understanding how to identify and calculate with a limiting reactant is crucial for predicting reaction outcomes and optimizing chemical processes. This guide will walk you through the steps necessary to tackle limiting reactant problems, ensuring you have a solid foundation to succeed in solving complex stoichiometry questions.
Step 1: Write and Balance the Chemical Equation
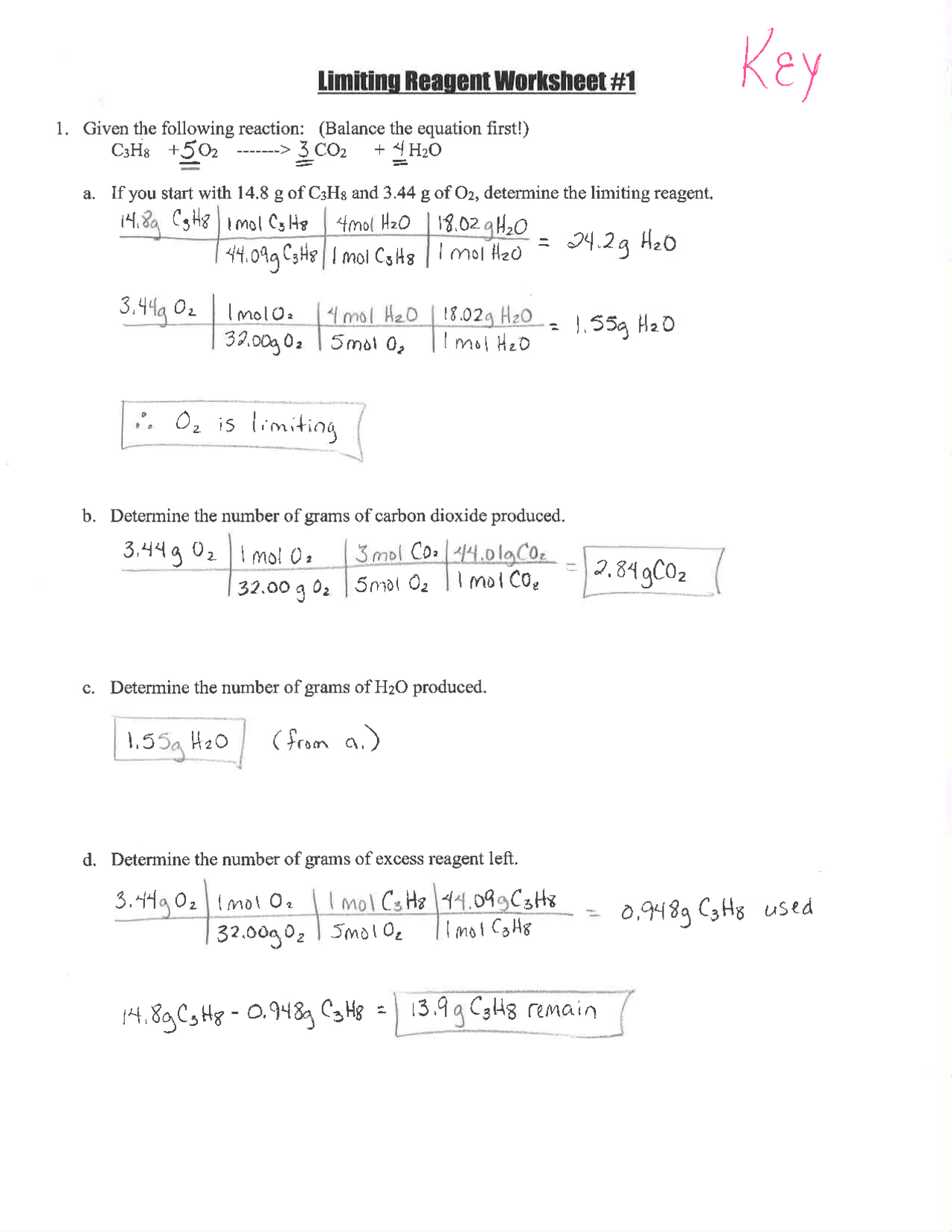
Before you can dive into calculations, it’s imperative to have a balanced chemical equation. This equation not only provides the relationship between reactants and products but also ensures that atoms are conserved. Let’s look at an example:
Consider the synthesis of water from hydrogen and oxygen:
2 H2 + O2 → 2 H2O
🔎 Note: Ensure the equation is balanced before proceeding; you can balance it by adjusting the coefficients until the atoms of each element match on both sides.
Step 2: Calculate the Moles of Each Reactant

If you are given the mass or volume of reactants:
- Convert mass to moles using the molar mass of the substances.
- Convert volume of gases to moles using the ideal gas law or standard conditions.
For instance, if we have:
| Reactant | Mass (g) | Molar Mass (g/mol) | Moles |
|---|---|---|---|
| H2 | 20 | 2 | 10 |
| O2 | 80 | 32 | 2.5 |

Step 3: Determine the Mole Ratio

Look at the balanced equation to see the stoichiometric ratio of the reactants. In our example, the ratio of H2 to O2 is 2:1.
Step 4: Identify the Limiting Reactant

The limiting reactant is determined by comparing the actual mole ratio of reactants with the stoichiometric ratio. Using our H2 and O2 example:
- We need 2.5 moles of O2 for every 10 moles of H2, but we only have 2.5 moles of O2.
- This means O2 is the limiting reactant because there’s not enough to react with all the H2.
Step 5: Calculate the Amount of Product Formed
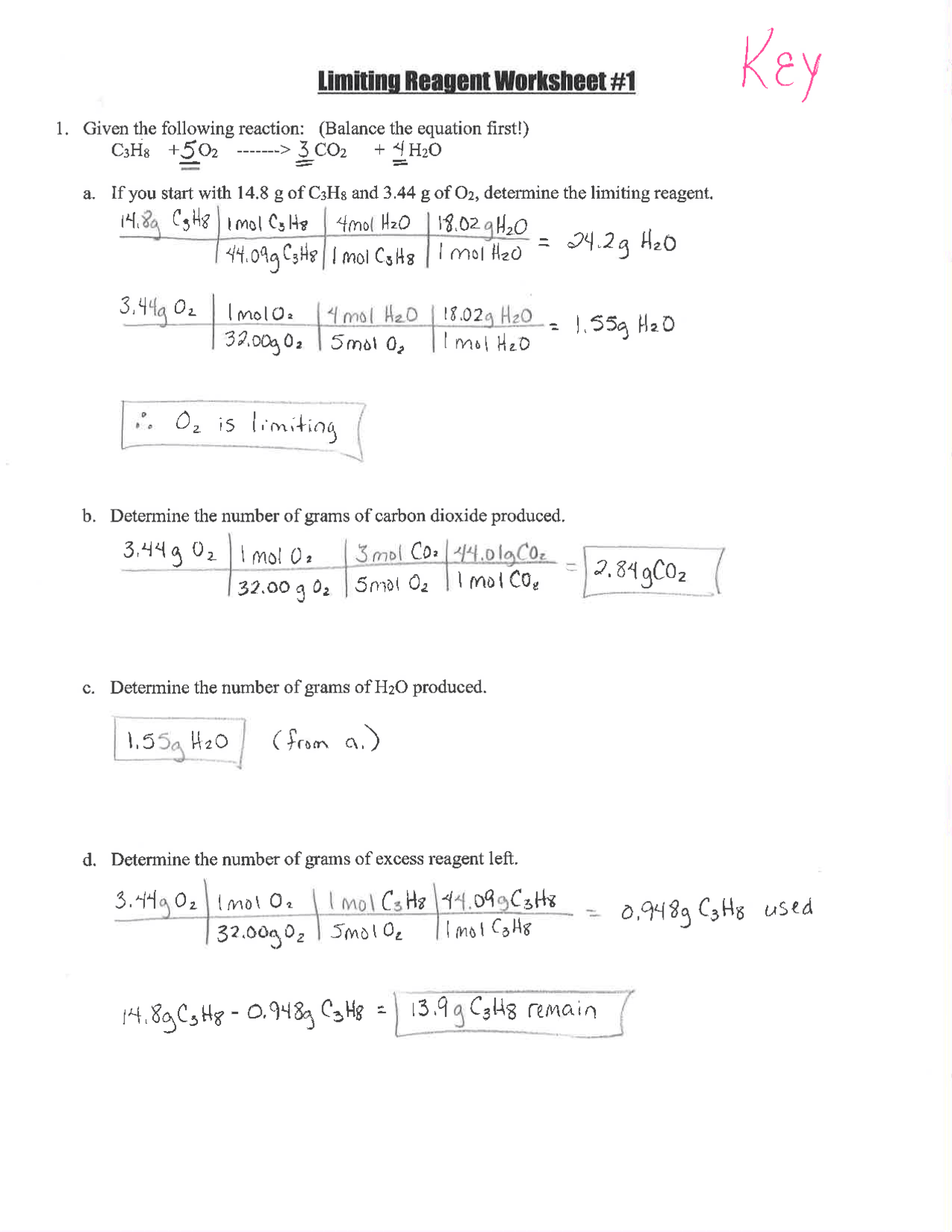
Using the limiting reactant, calculate the theoretical yield of the product:
To form water, we have:
- 2.5 moles of O2 can produce 5 moles of H2O, according to the balanced equation.
🔑 Note: The amount of product is directly proportional to the moles of the limiting reactant; always start with the limiting reactant to get the maximum yield.
Step 6: Adjust for Percentage Yield (if necessary)

Sometimes, not all of the limiting reactant reacts to form products due to experimental losses or inefficiencies. If a percentage yield is given or known:
- Multiply the theoretical yield by the percentage yield divided by 100 to get the actual yield.
The Importance of Limiting Reactants in Real-World Applications

Knowing which reactant is limiting is not just an academic exercise. In industries like pharmaceuticals, food processing, and manufacturing:
- Determining the limiting reactant helps in optimizing production processes by ensuring no resources are wasted.
- It allows chemists to predict the maximum yield possible from the available raw materials, which is critical for cost management and efficiency.
- In chemical synthesis, understanding the limiting reactant can also guide decisions on which reactants should be in excess to promote a desired reaction pathway.
Practical Tips for Solving Limiting Reactant Problems

- Always check your work: Ensure your calculations are correct, especially when converting units and dealing with stoichiometric ratios.
- Think about real-world implications: Even if you aren’t solving a problem in a lab, consider how your findings might impact real-world scenarios, like environmental control or industrial chemical processes.
- Use dimensional analysis: This method can simplify your calculations and help you track units, which is crucial in stoichiometry.
- Conceptual understanding: Always have a conceptual grasp of why a certain reactant is limiting, not just how to calculate it.
Understanding the process of identifying a limiting reactant, calculating theoretical yields, and considering real-world applications provides a comprehensive toolkit for addressing stoichiometry issues. Whether you're a student, teacher, or working professional in the field, this guide is designed to empower you with both the technical skills and the conceptual framework to tackle limiting reactant problems effectively.
What is the significance of balancing a chemical equation in stoichiometry?

+
Balancing a chemical equation ensures that the law of conservation of mass is observed, which states that matter cannot be created or destroyed in a chemical reaction. This balance allows for accurate determination of the moles of reactants and products, which is crucial for stoichiometric calculations, including finding the limiting reactant.
Why might experimental yield be different from theoretical yield?

+
Experimental yield can differ from theoretical yield due to several factors including: reaction inefficiencies, loss of product during handling or transfer, side reactions that produce different products, impurities in the reactants, or incomplete reactions. The percentage yield accounts for these discrepancies.
Can a limiting reactant ever change during a reaction?

+
Once a reaction starts, the limiting reactant does not change. However, if more of a reactant is added during the reaction, the original limiting reactant could potentially become in excess, and the new limiting reactant would be whatever is still fully consumed first. This dynamic is less common but possible in continuous flow or multi-step reactions.



