5 Steps to Ace Lewis Structures: Worksheet Answers Included
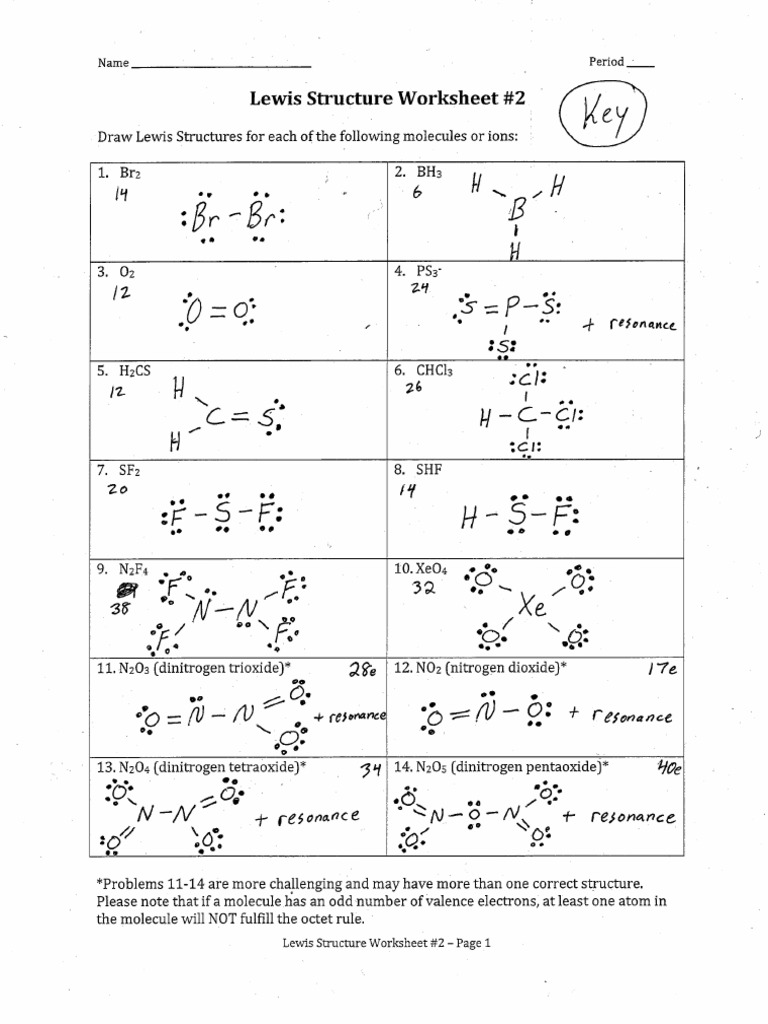
Mastering Lewis structures is an essential skill for any chemistry student or enthusiast. These structures help visualize the bonds between atoms and the placement of lone pairs in molecules, providing deep insights into the nature of chemical bonds. This blog post will guide you through five foundational steps to ace Lewis structures, complete with worksheet answers to reinforce your understanding.
Understanding Lewis Structures

Before diving into the steps, let’s first understand what Lewis structures are:
- They are diagrams that show the bonding between atoms in a molecule.
- They represent the distribution of valence electrons for each atom, focusing on octet stability (except for H, He, and other exceptions).
- These structures help predict the shape of a molecule, its polarity, and potential chemical reactivity.
🎓 Note: Lewis structures are primarily used for molecules with covalent bonds.
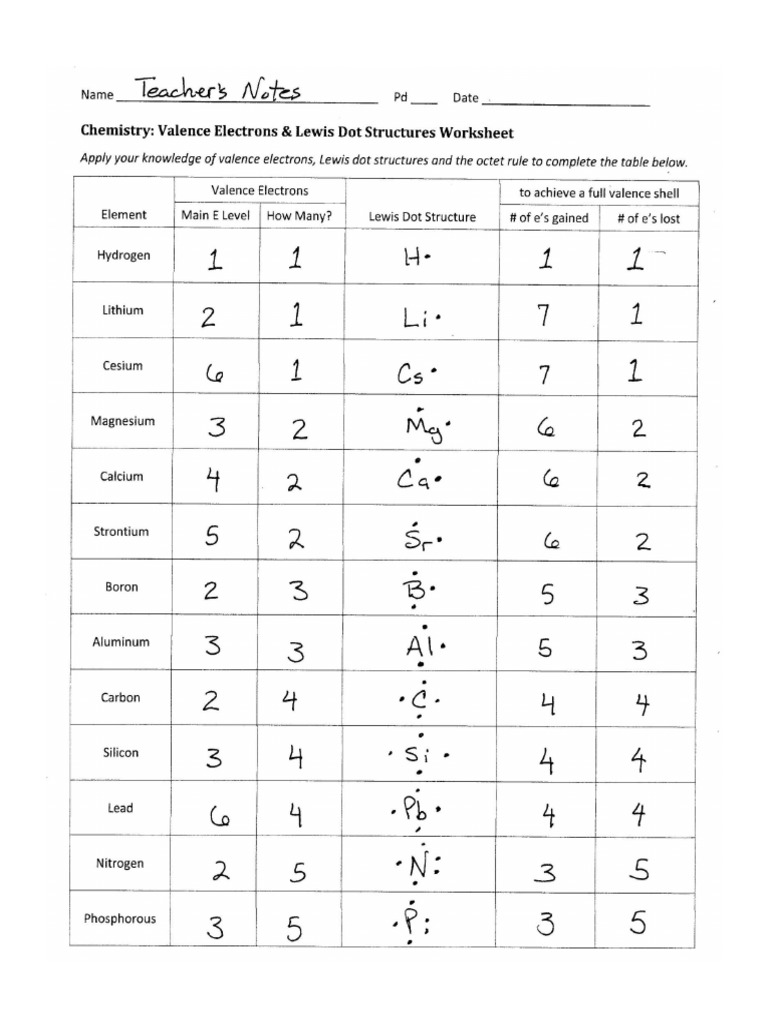
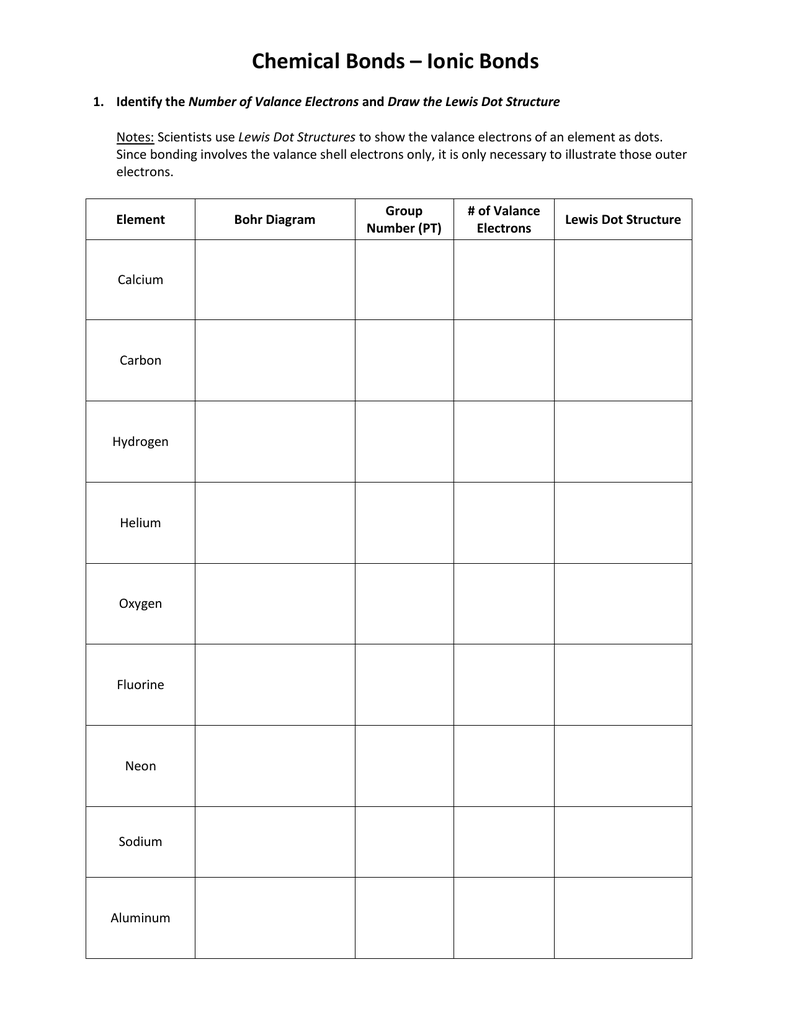
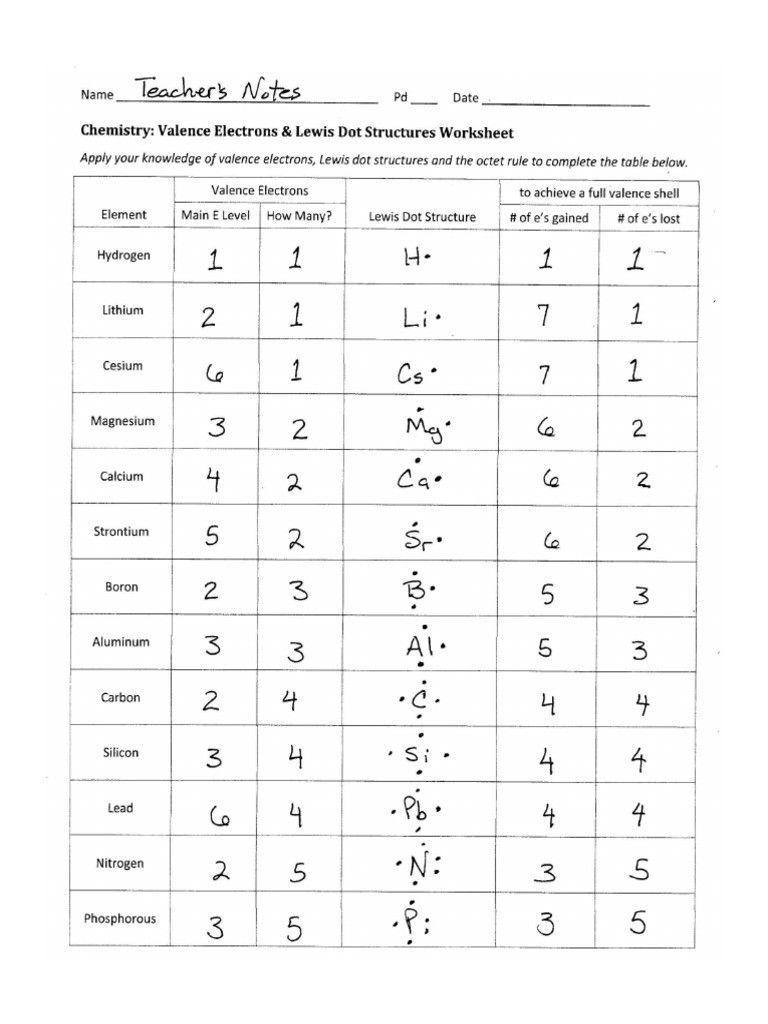
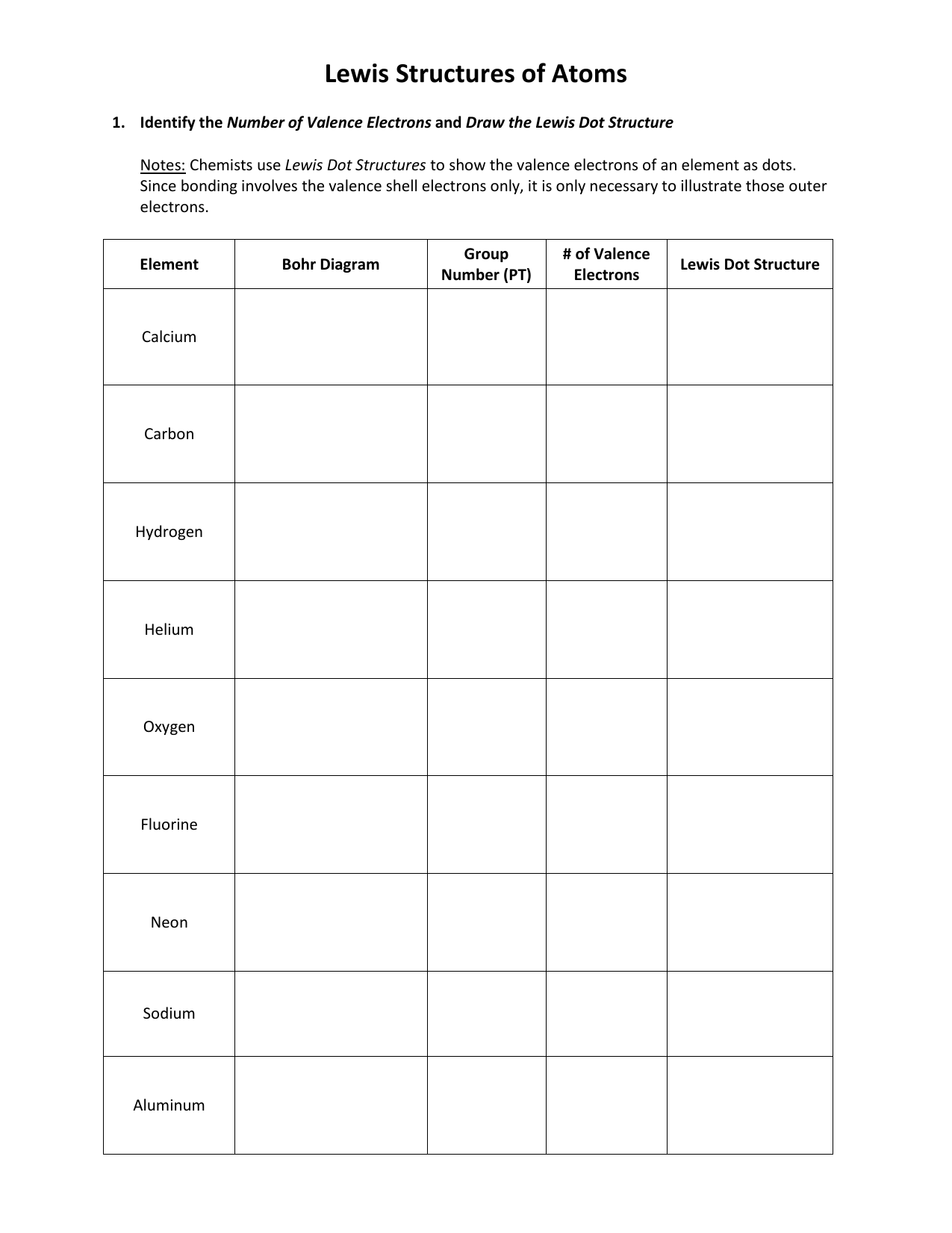
Step 1: Count Total Valence Electrons

Your first step towards mastering Lewis structures is to accurately count the number of valence electrons available for bonding. Here’s how:
- Identify the group number of each atom in the molecule from the periodic table.
- For main group elements, the group number equals the number of valence electrons.
- Include the charge of the molecule or ion when necessary. For cations, subtract electrons, and for anions, add electrons.
| Element | Group Number | Valence Electrons |
|---|---|---|
| Hydrogen | 1 | 1 |
| Carbon | 4 | 4 |
| Oxygen | 6 | 6 |

⚠️ Note: Noble gases (Group 18) have 8 valence electrons except for Helium, which has 2.
Step 2: Determine the Central Atom

Choosing the central atom is crucial because it will dictate how you arrange the Lewis structure:
- Usually, the atom with the lowest electronegativity and the highest number of bonding options takes this role.
- Examine the periodic table for exceptions; for instance, in H2O, hydrogen cannot be the central atom as it can only form one bond.
💡 Note: In simple cases, carbon is often the central atom due to its tetravalency.
Step 3: Draw Single Bonds

With the central atom in place, start connecting the other atoms via single bonds:
- Use lines to represent these bonds, remembering each line accounts for two electrons (one shared pair).
- Place hydrogen atoms at the periphery, as they only form one bond.
- Check your structure against the octet rule or exceptions for the second-period elements.
Step 4: Complete Octets and Add Lone Pairs
Now, distribute the remaining electrons to achieve octet configurations:
- Place lone pairs on the outer atoms first to satisfy their octet needs.
- Use any remaining electrons to complete the octet of the central atom. If there are not enough electrons, consider double or triple bonds or the presence of an odd number of valence electrons.
Remember, some elements like nitrogen (N) and sulfur (S) can expand their octet if necessary.
Step 5: Check Formal Charges and Adjust if Needed

To finalize your Lewis structure, calculate formal charges to ensure the structure is the most stable:
- Use the formula: Formal Charge = Valence Electrons - (Unshared Electrons + 1⁄2 Shared Electrons).
- If formal charges are high or unevenly distributed, consider adjusting the structure with additional bonds or lone pairs.
🔍 Note: A lower formal charge generally means a more stable structure, but not always.
Following these steps, you can create accurate Lewis structures for various molecules. Here are some worksheet answers to solidify your understanding:
- Example 1: Draw the Lewis structure for CO2.
- Example 2: Sketch the Lewis structure for H2O.
- Example 3: Construct the Lewis structure for NH3.
In wrapping up, mastering Lewis structures involves understanding electron distribution, following a systematic approach, and being aware of exceptions. By accurately determining valence electrons, selecting the correct central atom, connecting atoms appropriately, completing octets, and checking formal charges, you're on your way to confidently drawing Lewis structures for a wide array of compounds.
Why do we use Lewis structures?
+
Lewis structures help visualize the bonding and non-bonding electron pairs, giving insights into molecular geometry, polarity, and reactivity.
Can all atoms satisfy the octet rule in Lewis structures?
+
No, elements beyond the second period can have an expanded octet, and some smaller elements like hydrogen only require 2 electrons for stability.
How do formal charges relate to stability?

+
Structures with lower formal charges are typically more stable, but this is not an absolute rule as resonance or molecule-specific exceptions can exist.
What is the significance of single, double, and triple bonds in Lewis structures?
+
Bonds represent different levels of electron sharing; single bonds share one pair, double bonds share two pairs, and triple bonds share three pairs of electrons.