5 Steps to Master Lewis Dot Structures Easily

In the fascinating world of chemistry, Lewis dot structures, also known as electron dot structures or Lewis structures, are fundamental in understanding the electronic configuration of molecules. This visual representation allows chemists to predict the shape of molecules, understand chemical reactivity, and determine molecular polarity. Here's how you can master the technique of drawing Lewis dot structures in five straightforward steps.
Step 1: Count the Total Number of Valence Electrons

Begin by identifying the valence electrons for each atom in the molecule. Valence electrons are the electrons in the outermost shell which are involved in chemical bonding. Here’s how:
- Use the periodic table to find the number of valence electrons for each atom. Groups 1-2 and 13-18 have the number of valence electrons in their group number.
- Add up the valence electrons of all atoms involved in the molecule. If it’s an anion or cation, adjust the total by adding or subtracting electrons respectively.
Step 2: Choose a Central Atom
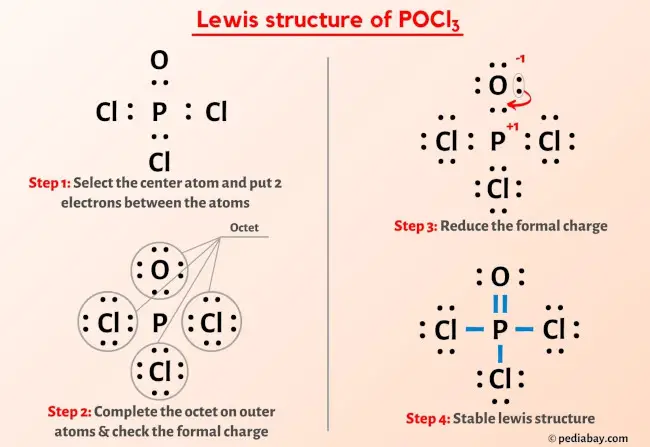
Select the central atom for your structure:
- Generally, the least electronegative atom (excluding hydrogen) is chosen as the central atom because it can form more bonds.
- If no single element is less electronegative than the others, choose one that appears most frequently or is most likely to form multiple bonds.

Step 3: Distribute the Electrons Among Atoms

Follow these steps to distribute electrons:
- Place the central atom and connect each other atom to it with a single bond.
- Count the electrons used in bonding. Each bond uses two electrons.
- Distribute the remaining electrons to complete the octet (or duet for hydrogen) of each outer atom.
- Any remaining electrons are placed around the central atom.
Step 4: Check for Formal Charges and Adjust

Ensuring all atoms have stable configurations:
- Calculate formal charges for each atom: Formal Charge = (valence electrons) - (lone pair electrons + half the bonding electrons).
- If the formal charge isn’t at its minimum, adjust bonds or lone pairs. Sometimes double or triple bonds are needed to reduce formal charges.
Step 5: Evaluate Resonance Structures

If there are multiple ways to draw the Lewis structure with the same atoms, consider resonance:
- Resonance structures occur when electrons can be delocalized over several atoms, sharing the electron load.
- Each structure contributes to the actual structure of the molecule, with some structures contributing more than others based on formal charge and stability.
💡 Note: Not all molecules have resonance structures, but recognizing when they do can be key in understanding the molecule's behavior.
Understanding these five steps provides a foundation for drawing Lewis dot structures, which is crucial for predicting molecular properties like bonding, shape, and reactivity. This knowledge can be applied to more complex problems in organic chemistry, biochemistry, and materials science, making it an invaluable skill for any chemist.
Why is it important to understand Lewis dot structures?

+
Understanding Lewis dot structures helps in predicting molecular shapes, understanding chemical reactivity, and explaining bonding behavior, which are essential in various chemical disciplines.
Can any molecule have resonance structures?

+
Not all molecules have resonance structures. Only molecules where multiple Lewis structures can be drawn, which differ only in the placement of electrons, exhibit resonance.
What do I do if the number of valence electrons isn’t even?

+
If the number of valence electrons is odd, the molecule will have at least one unpaired electron, which is typical for free radicals or molecules with an odd number of electrons.