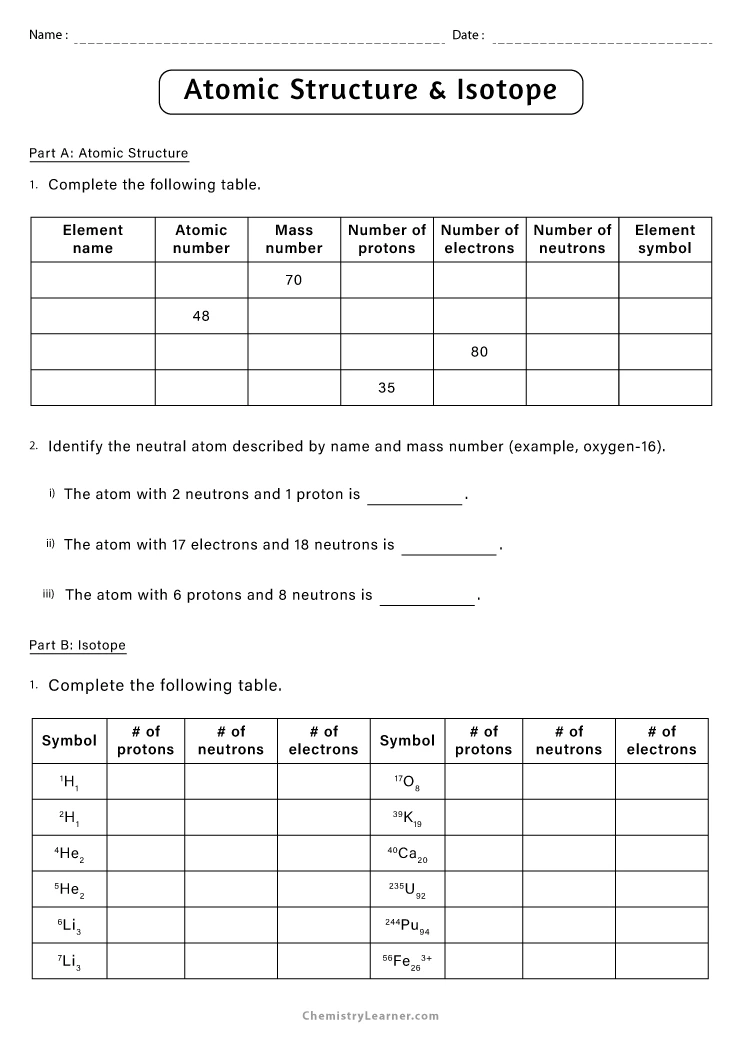
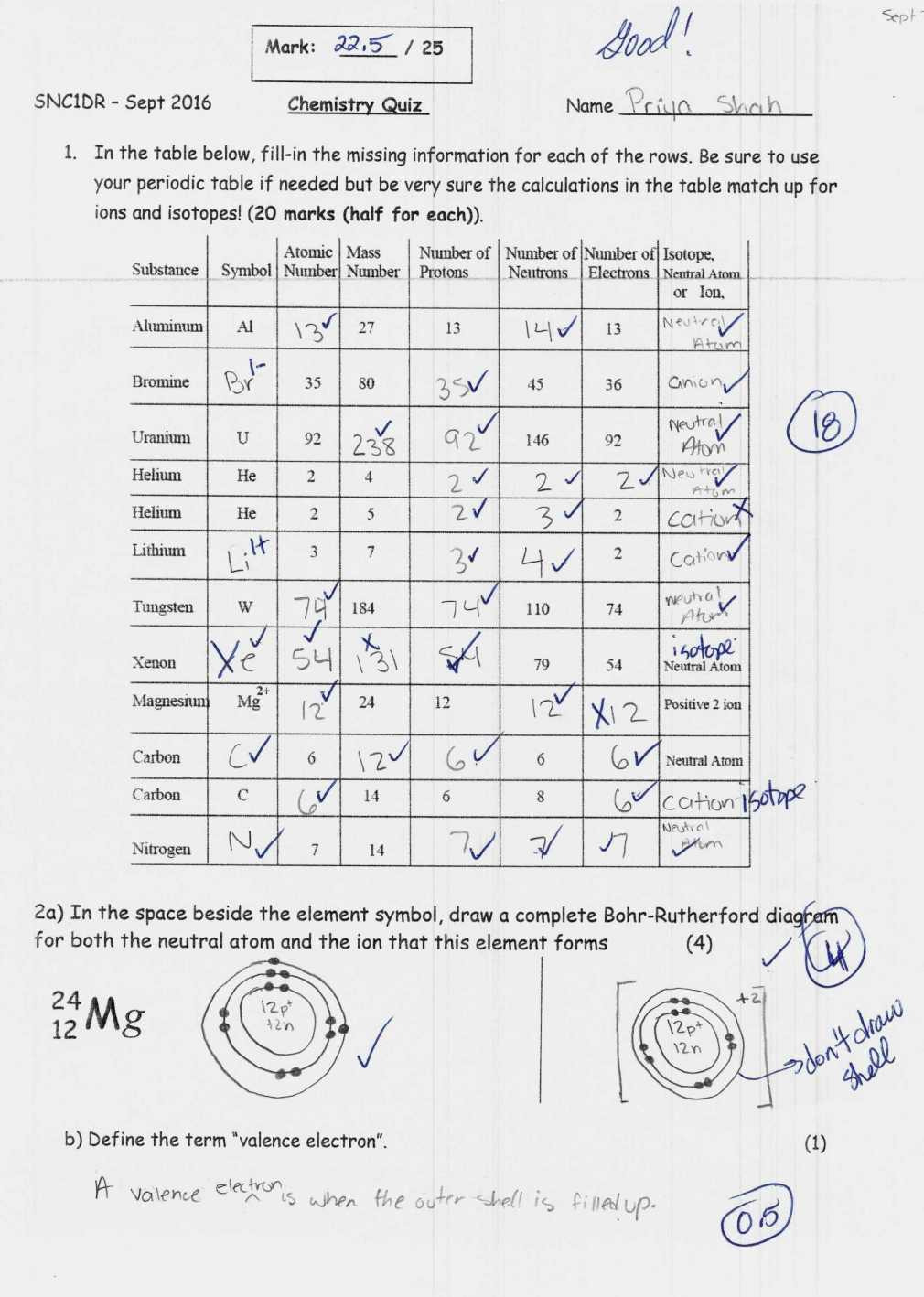
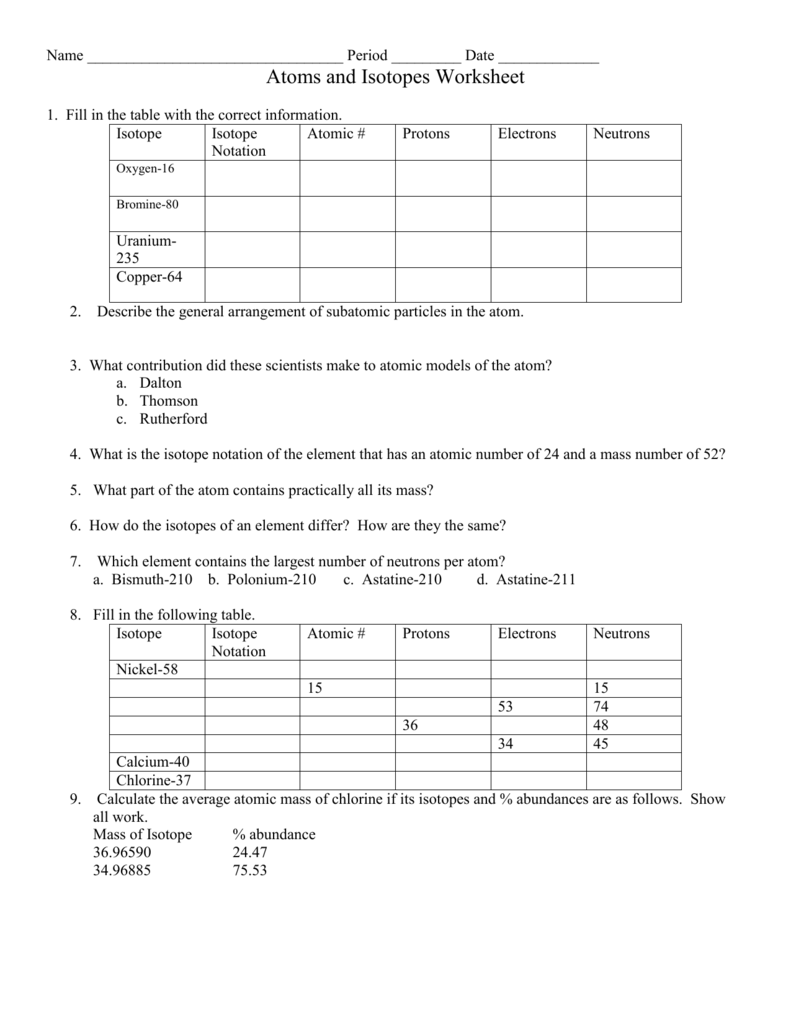
Master Isotopes and Ions: Practice Worksheet Guide

The journey through chemistry can often be an enthralling yet complex experience. Among the myriad of concepts students grapple with, understanding isotopes and ions stands out as particularly crucial for a comprehensive grasp of chemical fundamentals. This guide aims to provide an in-depth exploration into these essential concepts through a practice worksheet, designed to solidify your understanding and sharpen your skills.
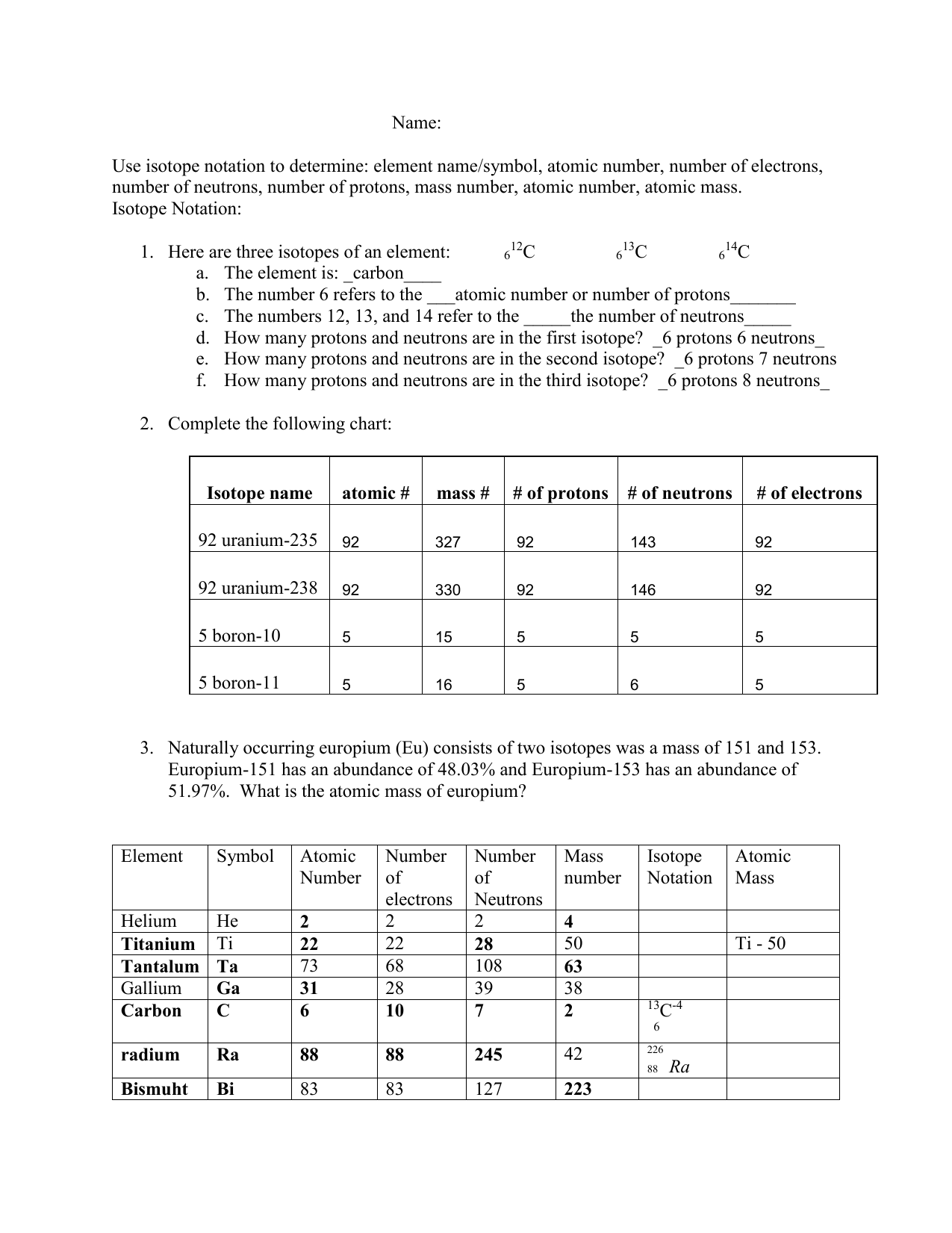
Understanding Isotopes

Isotopes are versions of an element that possess different numbers of neutrons, thus altering their mass number while retaining the same atomic number. Here’s how to identify and differentiate isotopes:
- Atomic Number (Z): This remains constant for all isotopes of an element. It indicates the number of protons in an atom's nucleus.
- Mass Number (A): This changes with isotopes. It is the total number of protons and neutrons. The difference in mass number among isotopes is due to varying neutron counts.

Practice Exercises for Isotopes

To get a firm grasp on isotopes, engage in the following exercises:
| Element | Atomic Number | Mass Number | Number of Neutrons |
|---|---|---|---|
| Hydrogen | 1 | 1, 2, 3 | 0, 1, 2 |
| Carbon | 6 | 12, 14 | 6, 8 |
| Oxygen | 8 | 16, 17, 18 | 8, 9, 10 |

⚠️ Note: When identifying isotopes, ensure the atomic number matches, but the mass number can differ due to the neutron count.
Understanding Ions
Ions are atoms or molecules that have gained or lost electrons, thus obtaining a net electric charge. Here’s a breakdown:
- Anions: Gain electrons, acquiring a negative charge. They are generally formed by non-metals.
- Cations: Lose electrons, gaining a positive charge. These are formed by metals.
Practice Exercises for Ions
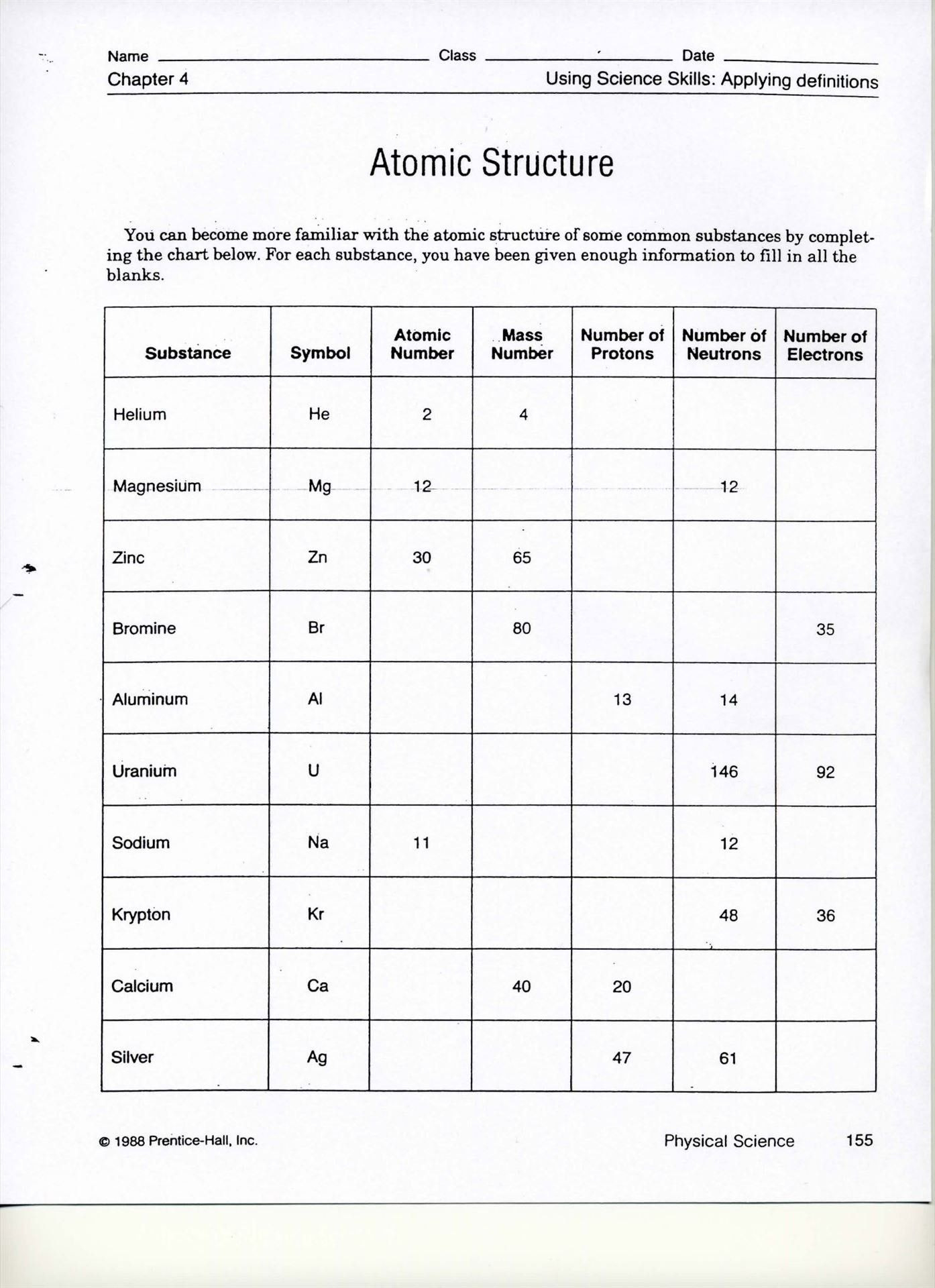
Here are some ion-related exercises:
- Calculate the charge of an atom with 11 protons and 10 electrons.
- Determine the number of electrons in a chloride ion (Cl⁻).
⚠️ Note: The charge of an ion can be calculated by subtracting the number of electrons from the number of protons.
Combining Isotopes and Ions

Now let’s delve into scenarios where isotopes can also form ions:
- Deuterium (H-2 or D): This isotope of hydrogen can lose an electron, forming D⁺, a positively charged ion.
- Carbon-14 (¹⁴C): This radioactive isotope of carbon can gain or lose electrons, creating charged particles.
Consider these practical applications:
- In medical imaging, positron-emitting isotopes like ¹⁸F can be used in PET scans, where the ions are key to their detection.
- Water (H₂O) can contain deuterium, thus forming heavy water (D₂O), which has implications in nuclear reactors.
Advanced Practice Exercises

Test your understanding with these advanced questions:
- What happens if carbon-12 (¹²C) gains an electron?
- What are the differences between ¹⁸O and ¹⁸O⁻?
Final Thoughts
Mastering isotopes and ions equips students with the knowledge to delve deeper into various fields of science, from nuclear physics to biochemistry. These exercises are designed not only to enhance your understanding of these topics but also to improve your problem-solving skills and conceptual clarity. Always remember that isotopes and ions are not just theoretical constructs but have real-world applications that can profoundly impact both science and society.
What is the difference between an isotope and an ion?

+
An isotope is a form of an element with a different number of neutrons, changing the mass number but not the atomic number. An ion, however, has gained or lost electrons, thus acquiring a charge.
Why are isotopes important in medicine?
+
Isotopes, particularly those that are radioactive, are used in diagnostic imaging like PET scans to visualize metabolic activity within the body, aiding in the detection of diseases.
Can an ion be an isotope?
+
Absolutely, as atoms can exist in various isotopic forms, any atom can potentially lose or gain electrons, forming an ion. Thus, an isotope can become an ion.