Chemical Reactions Basics: Free Printable Worksheet for Learning

Chemical reactions are the heartbeat of chemistry, the secret behind everything from cooking your dinner to building the car you drive. They are dynamic processes that involve the transformation of substances, known as reactants, into different substances, known as products. Understanding these reactions is fundamental for anyone interested in the sciences, and there's no better way to learn than by doing. This comprehensive guide provides a free printable worksheet to help students and enthusiasts alike delve into the fascinating world of chemical reactions.
What Are Chemical Reactions?

At its core, a chemical reaction involves the rearrangement of atoms. Here's what you need to know:
- Reactants: The starting substances in a chemical reaction.
- Products: The resulting substances after the reaction has taken place.
- Energy Changes: Reactions either release energy (exothermic) or absorb it (endothermic).
- Types of Reactions: There are various types like synthesis, decomposition, single displacement, double displacement, and combustion.

The Importance of Learning Chemical Reactions
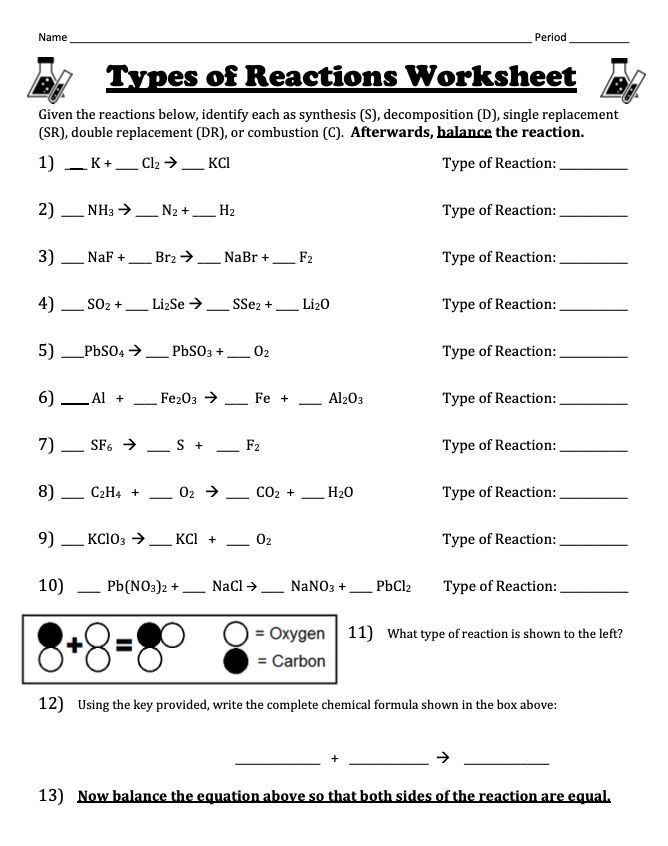
Chemical reactions are not just confined to the classroom; they impact our daily lives in numerous ways:
- Industrial Applications: From producing plastics to fuels, understanding reactions is crucial.
- Medical Field: Knowledge of biochemical reactions can lead to new treatments and drugs.
- Environmental Impact: Understanding reactions helps in managing pollutants and waste.
Free Printable Worksheet on Chemical Reactions

We've created a worksheet tailored to help you understand the basics:
Download Your Free Worksheet

Your journey to mastering chemical reactions starts with this easy-to-use worksheet. Here's how to use it effectively:
- Download and print the worksheet.
- Work through each section, focusing on the different types of reactions.
- Try to balance chemical equations, identify reaction types, and understand energy changes.
- Use the space provided to sketch reactions or jot down notes.
How to Use the Worksheet

Here are some tips to maximize your learning:
- Balancing Equations: Start with simple reactions and gradually increase complexity.
- Types of Reactions: Use the worksheet to familiarize yourself with different reaction categories.
- Energy Changes: Understand how reactions can either provide or require energy.
Exercise Examples
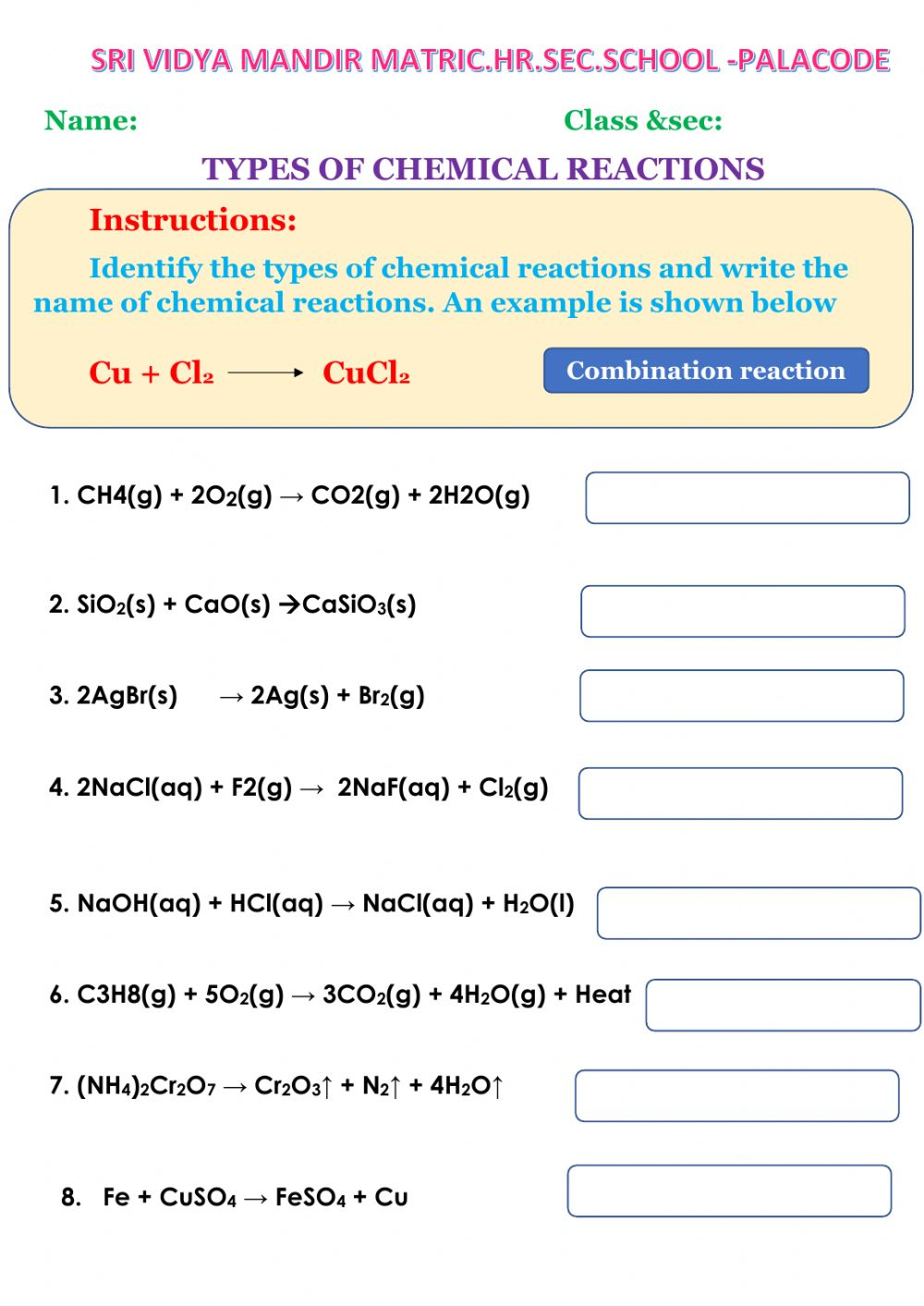
Here are some exercise examples you might encounter:
| Reactants | Products | Type of Reaction |
|---|---|---|
| H2O2 | H2O + O2 | Decomposition |
| NaCl + AgNO3 | AgCl + NaNO3 | Double Displacement |
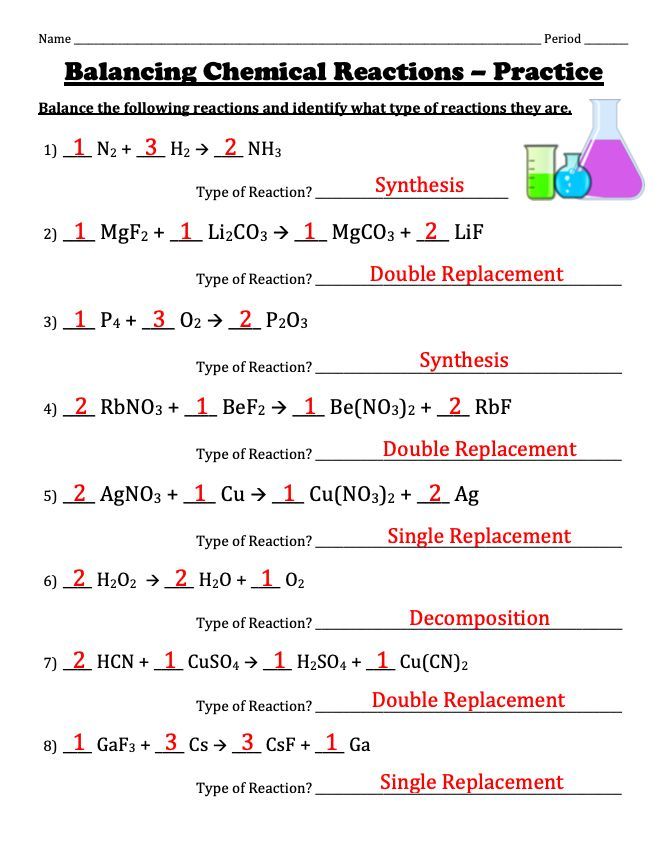
📝 Note: Keep in mind that balancing equations requires considering the law of conservation of mass. All atoms must be accounted for on both sides of the reaction arrow.
Balancing Chemical Equations

Balancing equations can seem daunting at first, but here are some steps to help you:
- Identify all elements and their initial counts in the reactants and products.
- Choose an element to balance first; typically, it's best to start with the most complex substance.
- Use coefficients to balance each element one at a time, revisiting elements if necessary.
- Check the final balance to ensure all atoms are accounted for.
Understanding Different Types of Reactions

Chemical reactions can be categorized into several types:
- Synthesis: A + B → AB
- Decomposition: AB → A + B
- Single Displacement: A + BC → AC + B
- Double Displacement: AB + CD → AD + CB
- Combustion: Often involves oxygen producing heat or light, like: CxHy + O2 → CO2 + H2O
🔥 Note: Combustion reactions are particularly important in energy production and environmental chemistry, as they are exothermic and involve heat release.
By now, you've had a deep dive into the fascinating world of chemical reactions. Understanding how elements combine, split apart, and exchange places is more than just academic; it's a window into the very essence of what makes our world work. With the tools provided in our free printable worksheet, you're well-equipped to explore and master these reactions. This journey through chemistry's fundamental processes not only enhances your knowledge but also shows you the real-world applications and the beauty of how chemistry can explain so much of the natural and man-made phenomena around us.
Why are chemical reactions important in daily life?

+
Chemical reactions are vital because they drive many everyday processes like digestion, cooking, cleaning, and even the way materials interact with the environment. They are the basis of industrial production, healthcare, and environmental sustainability.
How can I balance chemical equations easily?

+
Start by identifying the number of atoms of each element on both sides of the equation. Begin with the element that appears least frequently. Use coefficients to balance, ensuring you respect the conservation of mass, and double-check your work.
What are some real-life examples of chemical reactions?
+
Examples include:
- Rusting of iron (oxidation)
- Baking a cake (chemical change of ingredients)
- Burning of wood or gasoline (combustion)
- Digestion (hydrolysis and other metabolic reactions)
Can I perform experiments based on the worksheet exercises?

+
Yes, many of the exercises can be safely replicated in a controlled environment. However, always use proper safety equipment, follow guidelines, and ensure adult supervision if needed.



