5 Key Concepts for Understanding Acids and Bases

Understanding the behavior and properties of acids and bases is fundamental in the study of chemistry. These concepts not only provide insights into how chemical reactions occur but also underpin many industrial processes, environmental issues, and biological functions. Here are five key concepts that are essential for anyone looking to grasp the chemistry of acids and bases:
The Nature of Acids and Bases
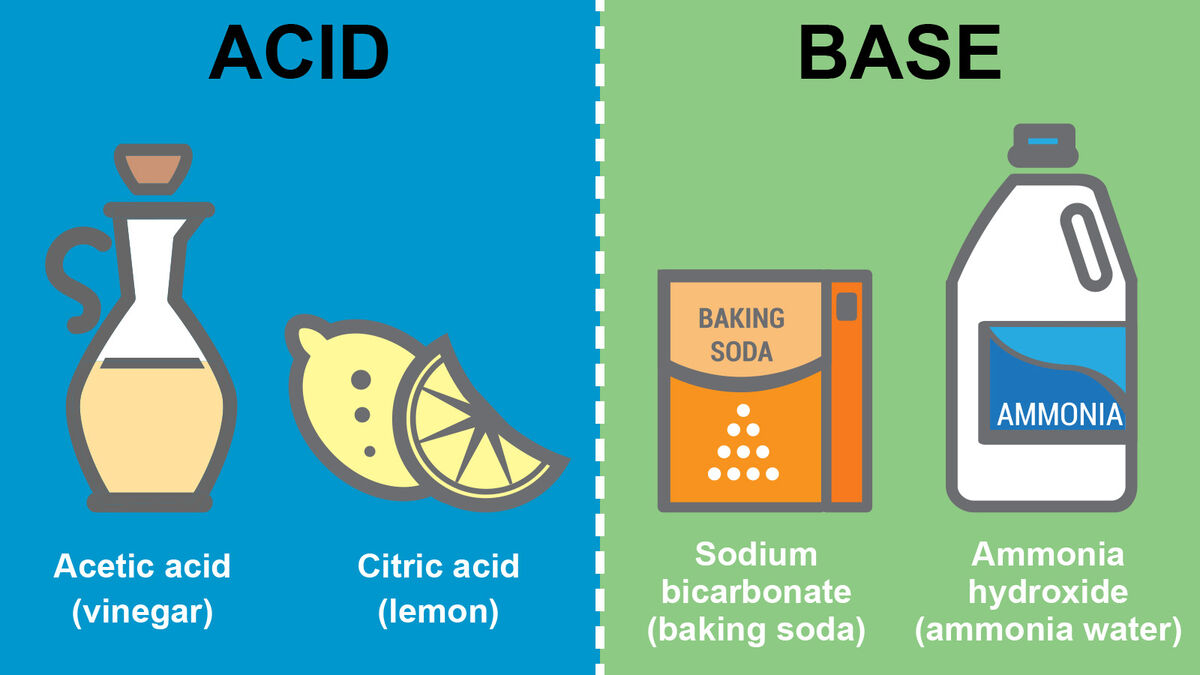
The classical understanding of acids and bases comes from:
- Arrhenius Theory: Acids release hydrogen ions (H+) in solution, and bases release hydroxide ions (OH-).
- Brønsted-Lowry Theory: An acid is a proton (H+) donor, while a base is a proton acceptor.
- Lewis Theory: An acid is an electron-pair acceptor, and a base is an electron-pair donor.
Each theory has its nuances and applies in different contexts, offering a broad spectrum of understanding the acidity or basicity of substances.
Measuring Acidity: The pH Scale


The pH scale measures how acidic or basic a solution is. It ranges from 0 to 14, where:
- pH < 7 indicates acidity
- pH > 7 indicates alkalinity (basicity)
- pH = 7 is neutral (e.g., pure water)
The pH is calculated as:
pH = -log[H+]
This logarithmic scale means each whole pH value below 7 is ten times more acidic than the previous one, and above 7, each whole value is ten times more basic.
Conjugate Acid-Base Pairs
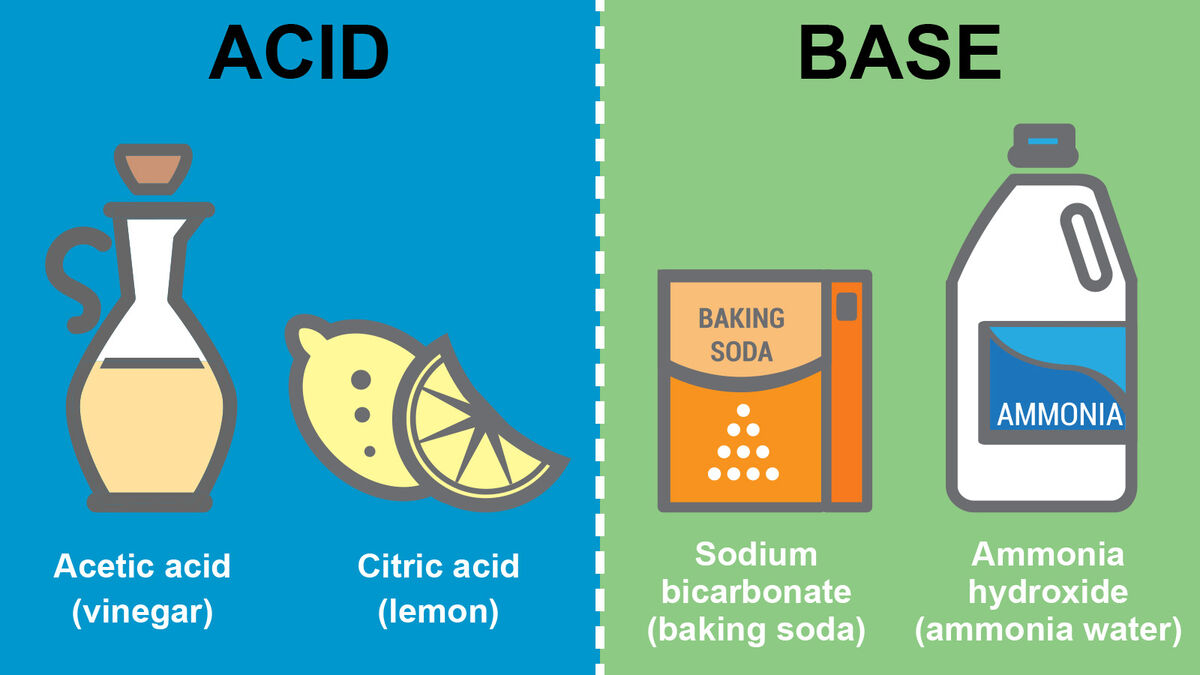
Every acid has a conjugate base, and every base has a conjugate acid. This pair exists because:
- When an acid donates a proton, what's left is the conjugate base.
- When a base accepts a proton, it becomes its conjugate acid.
Here's a simple example:
| Acid | Conjugate Base |
|---|---|
| HCl (Hydrochloric Acid) | Cl- (Chloride Ion) |
| H2O (Water) | OH- (Hydroxide Ion) |

This concept is crucial in understanding equilibrium in acid-base reactions.
Acid Strength and Equilibrium

Acid strength is gauged by:
- Ka - Acid dissociation constant. A higher Ka value means a stronger acid because it ionizes more completely in solution.
- pKa - The negative logarithm of Ka, where lower values indicate stronger acids.
⚠️ Note: Acid strength determines how the reaction will proceed and its effects on pH and reaction rate.
Acid-Base Indicators

Indicators are substances that change color in response to changes in pH:
- Phenolphthalein - Colorless in acidic solutions and pink in basic solutions.
- Litmus Paper - Turns red in acidic conditions, blue in basic.
Here's how you can visualize their behavior:
| Indicator | Color in Acid | Color in Base |
|---|---|---|
| Phenolphthalein | Colorless | Pink |
| Litmus Paper | Red | Blue |
These visual changes are key in titrations and other analytical methods where accurate pH measurement is necessary.
To sum up, understanding acids and bases involves recognizing different definitions of these substances, measuring their strength, identifying their conjugate pairs, and appreciating the dynamic equilibrium in their reactions. Each concept contributes to the comprehensive understanding of how substances behave when interacting with water or other chemical species. This knowledge not only satisfies curiosity but also has practical applications in fields like medicine, industry, and environmental science.
What are some common acids and bases found in everyday life?

+
Some common acids include citric acid (in citrus fruits), vinegar (acetic acid), and stomach acid (hydrochloric acid). Common bases include baking soda (sodium bicarbonate), soap, and chalk (calcium carbonate).
Why is the pH scale logarithmic?

+
The pH scale is logarithmic because hydrogen ion concentration can vary dramatically in solutions. A logarithmic scale allows for a wide range of concentrations to be expressed in a manageable numerical range from 0 to 14.
Can a substance be both an acid and a base?

+
Yes, substances like water can act as both acids and bases. This is known as amphotericity, where the substance can either accept or donate a proton depending on the surrounding chemical environment.


