5 Essential Tips for Mastering Heating Curves
Mastering heating curves is essential for students and professionals in chemistry, material science, physics, and many other scientific disciplines. A heating curve visually represents how the temperature of a substance changes over time as heat is applied. Understanding these curves not only helps in visualizing phase changes but also in predicting and controlling reactions and processes. Here are five crucial tips to master the art of interpreting and utilizing heating curves effectively.
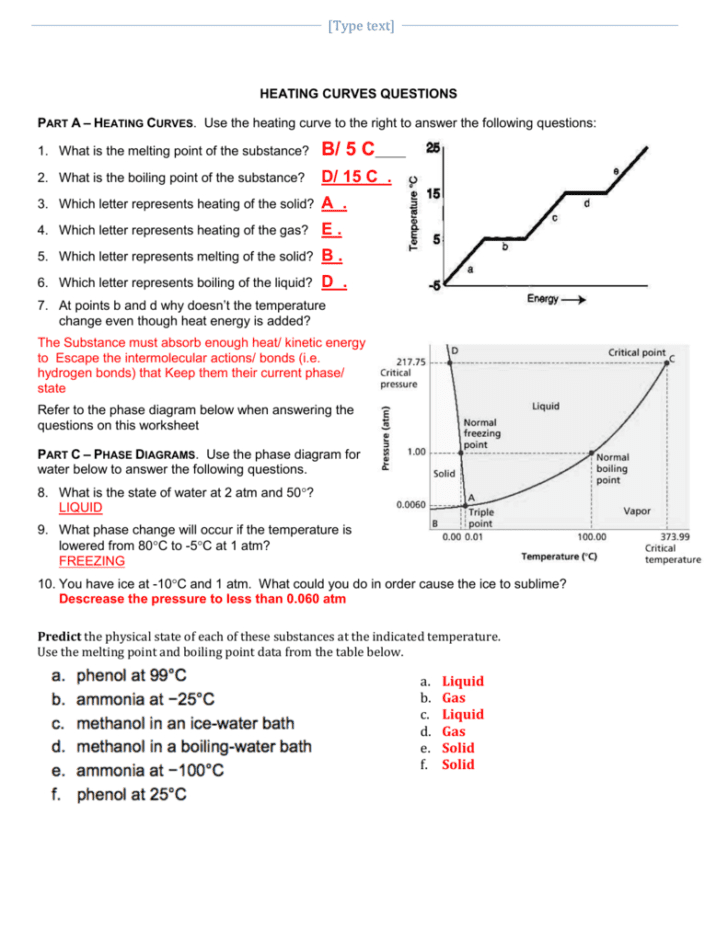
1. Understand the Phases and Phase Changes

The first step in mastering heating curves is to understand the different phases of matter: solid, liquid, and gas, and the transitions between them. A typical heating curve for a pure substance will have:
- Heating Zones: These are the flat parts of the curve where heat energy is added, but the temperature does not change. This energy is used to break or form bonds, allowing the substance to change its phase.
- Phase Change Zones: Here, the substance changes its state. These are marked by horizontal lines on the curve, representing melting (solid to liquid), boiling (liquid to gas), or sublimation (solid to gas).
Understanding these phases and changes helps in:
- Predicting the energy required for phase changes.
- Identifying the points where physical and chemical properties of the substance change.

2. Learn to Read the Curve for Heat of Fusion and Heat of Vaporization
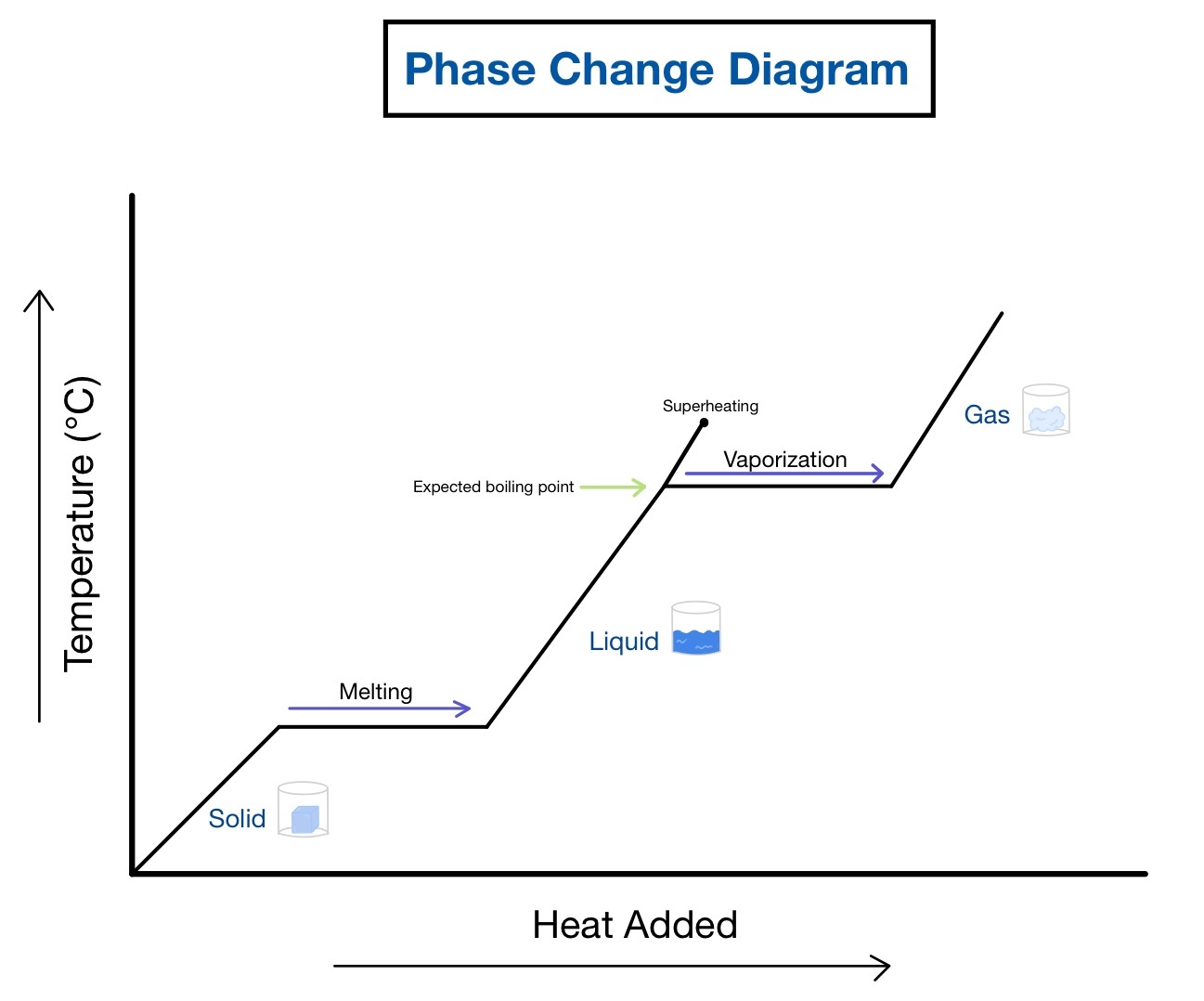
The heating curve provides insights into the heat of fusion and vaporization:
- Heat of Fusion: This is the energy required to change a substance from a solid to a liquid at its melting point. The longer the horizontal line on the curve at the melting point, the higher the heat of fusion.
- Heat of Vaporization: Similarly, this energy is needed to convert liquid into gas at the boiling point. The length of the horizontal line at the boiling point indicates the heat of vaporization.
💡 Note: Heat of fusion and vaporization are intrinsic properties and can be used to identify the substance if you don’t know what it is.
3. Recognize the Significance of Specific Heat Capacity

Each substance has a unique specific heat capacity, which is the amount of heat required to raise the temperature of one unit mass by one degree Celsius. Here’s how to utilize this in a heating curve:
| Phase | Description | Effect on Heating Curve |
|---|---|---|
| Solid | Solid-state heating | Steeper slope reflecting the specific heat of the solid |
| Phase Change | Transition to liquid | Horizontal line |
| Liquid | Liquid-state heating | Less steep slope compared to the solid phase, reflecting the liquid’s specific heat |
| Phase Change | Transition to gas | Horizontal line |
| Gas | Gas-state heating | Slope different from both liquid and solid phases |

Recognizing these differences allows for:
- Predicting how much energy is needed to heat substances to desired temperatures.
- Understanding why certain materials are better for heat storage or transfer.
4. Implement the Knowledge for Laboratory Analysis

In lab work, heating curves are crucial for:
- Purity Analysis: The sharpness of phase change lines can indicate the purity of a sample.
- Reaction Optimization: By knowing the heating curve, you can control the temperature during reactions for better yield.
- Material Processing: Understanding phase changes helps in controlling processes like casting, molding, or vapor deposition.

5. Utilize Software for Enhanced Learning and Analysis

Modern software tools can significantly enhance your understanding and utilization of heating curves:
- Simulation Software: Programs like PhET Interactive Simulations allow you to model heating curves for different substances, showing in real-time how energy impacts phase changes.
- Data Analysis: Software like Excel or Origin can be used to plot real data from experiments, providing insights into deviations from expected curves.
- Interactive Notebooks: Platforms like Jupyter Notebooks enable you to write code to simulate heating curves, allowing you to explore parameters easily.
Using such tools:
- Helps to visualize what’s happening at a molecular level.
- Enables experimentation with variables like pressure and impurities.
⚙️ Note: Software can simulate ideal conditions, but real-world experiments might deviate due to impurities, pressure changes, or measurement errors.
To truly master heating curves, one must combine theoretical knowledge with practical application. Remember, these curves are not just theoretical tools but practical tools for enhancing our understanding of matter and energy interactions. Whether you're in a lab setting, working on a project, or teaching students, knowing how to read, interpret, and utilize heating curves can elevate your scientific prowess. From predicting energy requirements for heating a substance to understanding phase transitions at a molecular level, these tips form the backbone of effective learning and application in the field of thermodynamics and beyond.
What is the difference between a heating curve and a cooling curve?

+
A heating curve shows the temperature changes of a substance as heat is added over time, while a cooling curve illustrates the temperature drop as heat is removed. Both curves can be used to observe phase transitions, but a cooling curve will show the changes in reverse order.
Why does the heating curve have flat lines?

+
The flat lines on a heating curve represent phase changes where the temperature remains constant because the added heat is used to break intermolecular forces, not to raise the temperature. This occurs during melting and boiling points.
Can impurities affect a heating curve?

+
Yes, impurities can broaden the phase change zones on a heating curve, making the lines less sharp and causing the substance to change phase over a range of temperatures rather than at a fixed point. This is due to the presence of different substances with varying melting or boiling points.