Heating Curve Worksheet 1 Answer Key: 5 Essential Insights
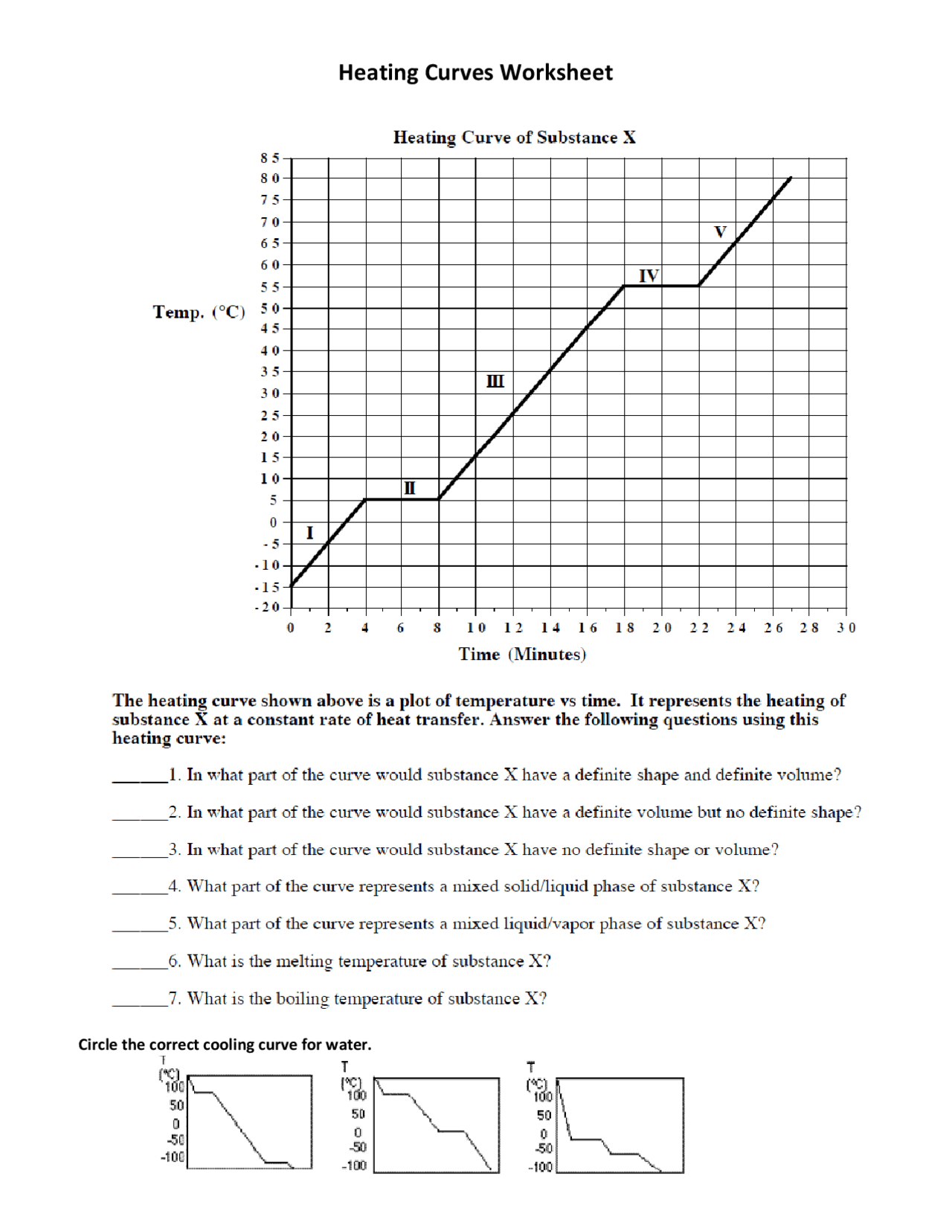
Understanding the concept of heating curves is crucial for students and professionals in the fields of chemistry, physics, and material science. A heating curve graphically represents the change in temperature of a substance as it absorbs heat at a constant rate. Here, we delve into the heating curve worksheet 1 answer key, providing five essential insights to help you master this fundamental concept.
1. Phases and Phase Changes

A heating curve typically goes through five distinct phases or stages:
- Solid Phase: Initially, the substance is in its solid form. Here, heat energy raises the temperature of the solid until it reaches its melting point.
- Melting (Fusion): At the melting point, the substance changes from a solid to a liquid. This phase is represented by a horizontal line on the curve where heat is absorbed without a change in temperature.
- Liquid Phase: Post-melting, the temperature again increases as heat is added, until the substance reaches its boiling point.
- Boiling (Vaporization): Similar to melting, here the substance changes from liquid to gas, again with no temperature change.
- Gas Phase: Once the substance is entirely in gas form, continued heating causes the temperature to rise further.
2. Interpreting the Curve
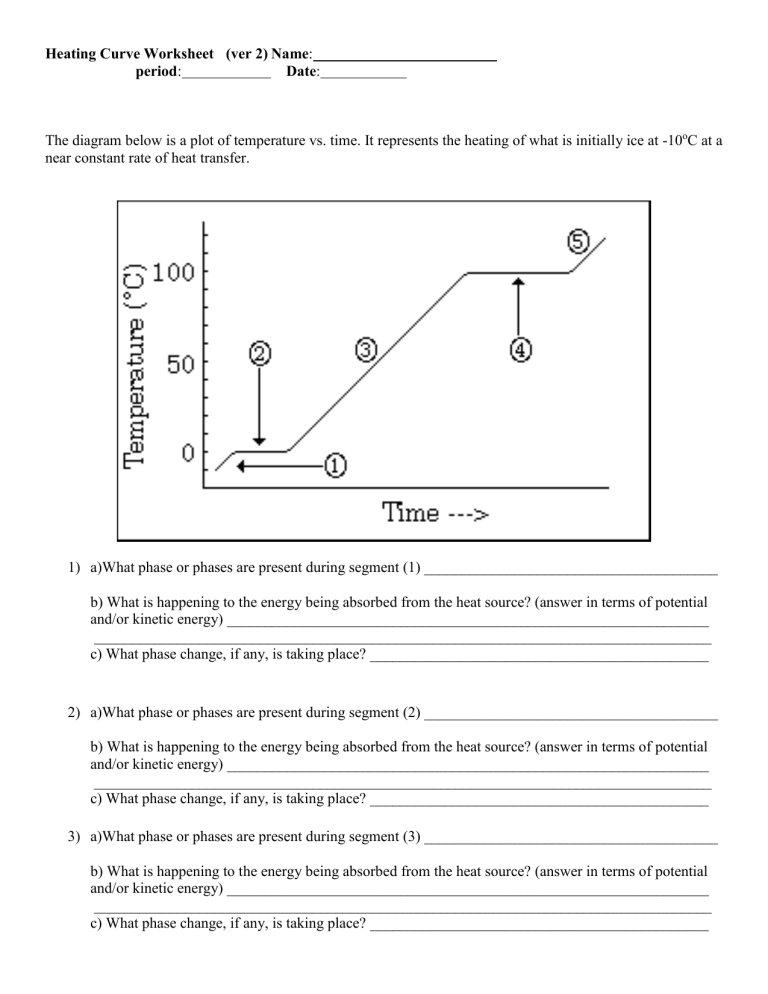
Interpreting a heating curve involves:
- Sloped Sections: These represent changes in temperature where the substance is either heating up or cooling down in its current phase.
- Flat Sections: Known as plateaus, these areas indicate phase changes where energy is used to change the substance’s state rather than increase its temperature.
- Energy Absorption: The amount of heat energy absorbed at each stage can be inferred by the length of time spent in each phase change.
3. Calculations from the Heating Curve

The heating curve worksheet often includes calculations for:
- Heat of Fusion (ΔHfus): The energy needed to transition from solid to liquid.
- Heat of Vaporization (ΔHvap): The energy required to convert liquid to gas.
- Heat Capacity ©: The amount of energy required to change the temperature of the substance by one degree.
🌟 Note: Ensure to use the specific heat capacity values for each phase of the substance when performing calculations.
4. Practical Applications

Understanding heating curves is not just theoretical; it has practical implications:
- Cooking and Food Processing: Knowing at what temperatures food melts or boils helps in culinary techniques like tempering chocolate.
- Material Engineering: Critical for melting metals or glass for manufacturing.
- Weather Forecasts: Predicting when and how ice will melt or when water will boil over different altitudes and conditions.
5. Troubleshooting and Common Misconceptions

Here are some common issues and misconceptions to watch out for:
- Phase Change Energy: Many assume that energy is not absorbed during phase changes, but energy is indeed used to break or form bonds, not increase temperature.
- Correct Heat Capacity Use: Using the heat capacity of one phase when the substance is in another phase is a common mistake.
- Curve Interpretation: Students sometimes mistakenly believe that during phase changes, the substance temperature continues to rise, whereas it remains constant.
In summary, mastering the heating curve allows us to understand how substances react to heat, which is pivotal in numerous scientific and practical applications. From cooking to engineering, from material synthesis to environmental science, a clear grasp of this concept enhances our ability to manipulate matter in controlled ways. Let’s continue exploring how we can apply this knowledge in various scenarios.
Why is there a plateau during phase changes in a heating curve?
+
The plateau represents the time when heat energy is being used to break molecular bonds or form new ones without increasing the temperature of the substance.
How do you calculate heat absorbed during different phases?

+
You use the formula Q = mcΔT for temperature changes in the same phase, and Q = mΔH for phase changes, where m is mass, c is specific heat capacity, ΔT is the temperature change, and ΔH is the heat of fusion or vaporization.
What does a steeper slope on the heating curve signify?
+
A steeper slope means that the substance is absorbing heat at a faster rate, leading to a quicker temperature rise.
Can heating curves be used for cooling processes?

+
Yes, a cooling curve is similar to a heating curve but shows how substances release heat to their surroundings, resulting in temperature decreases and phase changes from gas to liquid to solid.
What factors affect the shape of a heating curve?

+
Factors include the nature of the substance, atmospheric pressure, the rate of heat supply, and impurities in the substance.


