5 Essential Periodic Trends for Chemistry Students

In the vast expanse of chemistry, understanding periodic trends is crucial for students who aim to master the intricacies of the chemical elements. The periodic table, with its ordered elements, isn't just a static chart but a dynamic map that reveals how elements interact with each other due to their position on the table. This article will delve into five essential periodic trends that every chemistry student needs to understand. These trends are not only fundamental in predicting the properties of elements but also in explaining the chemical behavior, bonding, and reactivity.
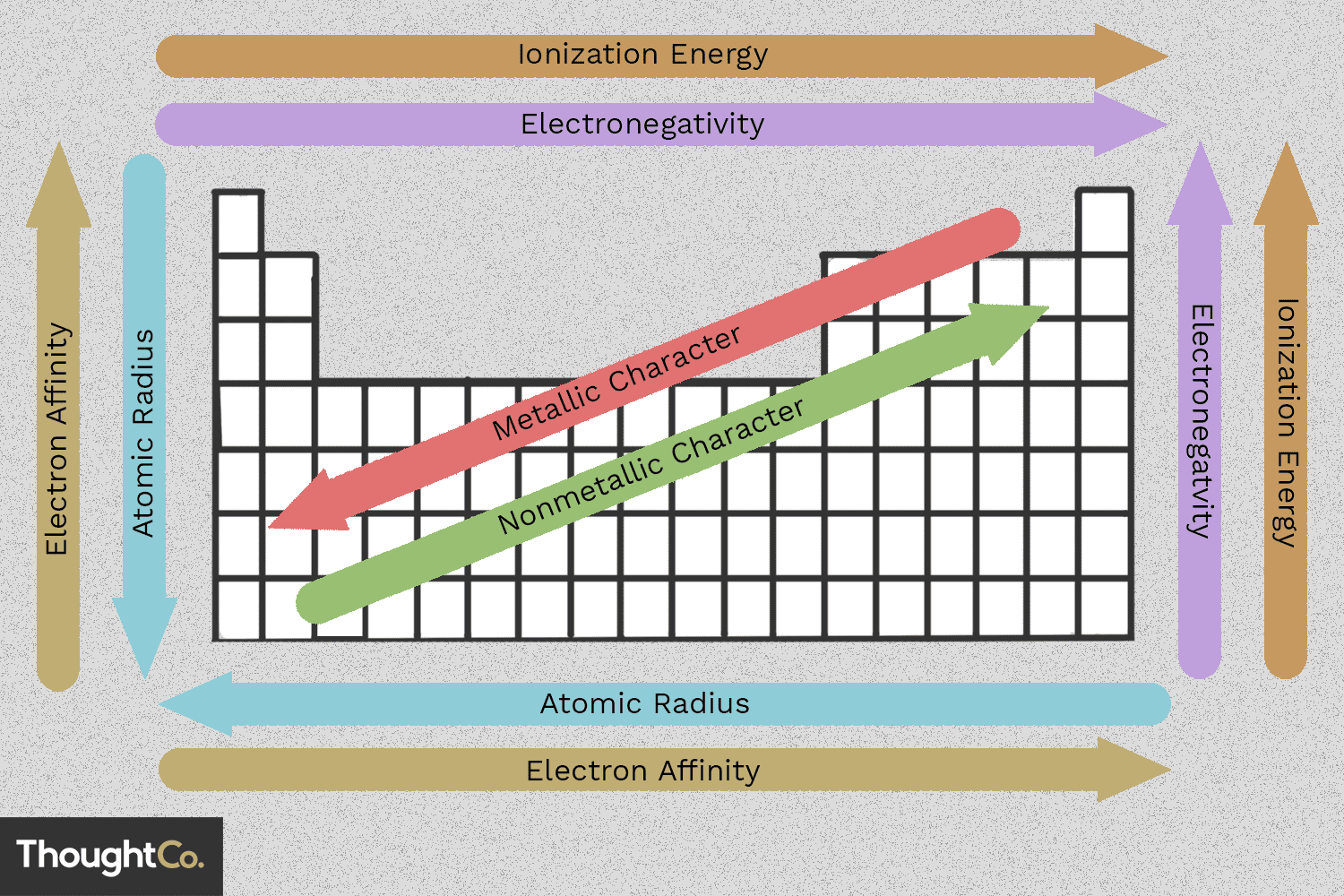
1. Atomic Radius

Atomic radius refers to the size of an atom. This trend across the periodic table is influenced by several factors:
- Effective Nuclear Charge: As you move across a period from left to right, the number of protons (nuclear charge) increases. This increased positive charge pulls electrons closer to the nucleus, decreasing the atomic radius.
- Shielding Effect: When moving down a group, the addition of electron shells increases. Electrons in inner shells shield outer electrons from the nucleus, thereby increasing the atomic radius due to less effective nuclear pull.

🔍 Note: While trends can be generalized, specific elements might deviate due to anomalies in electron configurations or atomic structure.
2. Ionization Energy

Ionization energy is the energy required to remove an electron from an atom in its gaseous state. This trend can be broken down as:
- Increase Across a Period: Moving left to right across the periodic table, ionization energy increases because electrons are closer to the nucleus due to higher effective nuclear charge, making them harder to remove.
- Decrease Down a Group: As we go down a group, atomic size increases due to more electron shells, which means electrons are further from the nucleus, reducing the energy needed to remove them.

3. Electron Affinity

Electron affinity is the measure of the energy change when an electron is added to a neutral atom to form a negative ion. The trends here are:
- General Increase Across Periods: Though there are exceptions, electron affinity generally increases from left to right due to increased nuclear charge, making it easier to add an electron.
- Irregular Decrease Down Groups: The addition of electron shells creates more distance between the electrons and nucleus, which can lower the electron affinity, but other factors like atomic structure play a role in this trend.
4. Electronegativity

Electronegativity indicates an atom’s ability to attract and hold onto electrons within a chemical bond. Here are the key points:
- Increases from Left to Right: Across periods, the trend in electronegativity follows a general increase due to increasing effective nuclear charge.
- Decreases from Top to Bottom: As atomic size increases down a group, the ability to attract electrons lessens, thus reducing electronegativity.

| Element | Electronegativity (Pauling Scale) |
|---|---|
| Hydrogen | 2.20 |
| Oxygen | 3.44 |
| Fluorine | 3.98 |

5. Reactivity

Reactivity is the tendency of an element to undergo chemical change, and its periodic trends are:
- Metals: Reactivity increases from right to left across periods and top to bottom down groups due to the ease of losing electrons.
- Non-metals: The trend inverts with reactivity increasing from left to right across periods and decreasing down groups, reflecting their affinity to gain electrons.
⚠️ Note: Reactivity doesn’t always follow these trends strictly due to specific electronic configurations or unique chemical reactions.
To summarize, understanding the periodic trends provides chemistry students with a powerful tool to predict chemical properties, reactions, and bonding behaviors. Whether it's the size of atoms, the energy required to remove or gain electrons, or how elements attract electrons in bonds, these trends underpin much of the predictability in chemistry. By mastering these trends, students can explain why elements behave the way they do, from the most reactive alkali metals to the inert noble gases.
Why do atomic radii decrease across periods?

+
Atomic radii decrease across periods because the effective nuclear charge increases, pulling electrons closer to the nucleus due to the higher number of protons. This inward pull outweighs the electron-electron repulsion.
What causes the trend in ionization energy?

+
Ionization energy trends are caused by the combined effect of nuclear charge and electron shielding. As effective nuclear charge increases across periods, it becomes harder to remove an electron. Conversely, down a group, electrons are farther from the nucleus due to shielding, requiring less energy.
How does electronegativity affect chemical bonding?
+
Electronegativity influences how electrons are shared in chemical bonds. A large difference in electronegativity between two atoms can lead to a more polar bond, potentially resulting in ionic bonding, while similar electronegativities lead to more covalent bonds.
Can you predict chemical reactivity using periodic trends?
+
Yes, periodic trends give a good indication of reactivity. For instance, metals in the far left of the periodic table are highly reactive as they easily lose electrons, while the trend for non-metals increases from left to right as they gain electrons more readily.
Are periodic trends absolute?

+
No, while trends provide a general framework, there are exceptions due to factors like electron configurations, anomalies in atomic structure, or other chemical behaviors.