Gas Laws Review Worksheet: Master Chemistry Concepts Easily

In the world of chemistry, understanding the behavior of gases is fundamental. Gas laws govern how gases react to changes in temperature, pressure, and volume, and they are critical for students and professionals alike in fields ranging from physics to engineering. This comprehensive guide will delve into various gas laws, providing a clear framework to master these essential chemistry concepts. Whether you are a high school student struggling with your chemistry class, a college student diving deep into physical chemistry, or an enthusiast, this blog post will make the gas laws seem less like a daunting maze of formulas and more like a structured set of guidelines for understanding the universe.
The Basics of Gas Laws

Gases, unlike solids and liquids, have no definite shape or volume, which makes them fascinating yet complex to study. Here are some fundamental concepts to start with:
- Boyle's Law: This law states that at constant temperature, the volume of a gas is inversely proportional to the pressure applied. Mathematically, P1V1 = P2V2.
- Charles's Law: At constant pressure, the volume of a gas is directly proportional to its temperature (in Kelvin). This can be expressed as V1/T1 = V2/T2.
- Gay-Lussac's Law: For a given mass and at constant volume, the pressure of a gas is directly proportional to its temperature. It's denoted as P1/T1 = P2/T2.
- Avogadro's Law: Equal volumes of all gases at the same temperature and pressure contain an equal number of molecules. This is why the volume of a gas (V) is proportional to the amount of substance (n), or V1/n1 = V2/n2.
📝 Note: Ensure temperatures are always converted to Kelvin when using gas laws.
The Combined Gas Law

The individual gas laws mentioned above can be combined into a single equation known as the Combined Gas Law. This law allows us to account for changes in pressure, volume, and temperature simultaneously, providing a more comprehensive understanding of gas behavior:
P1V1/T1 = P2V2/T2
Here’s how you might use this law:
- When you know initial conditions and want to predict final conditions.
- When any two conditions (like pressure and volume) change while the third (temperature) remains constant, you can rearrange the equation to fit the known and unknown variables.
The Ideal Gas Law
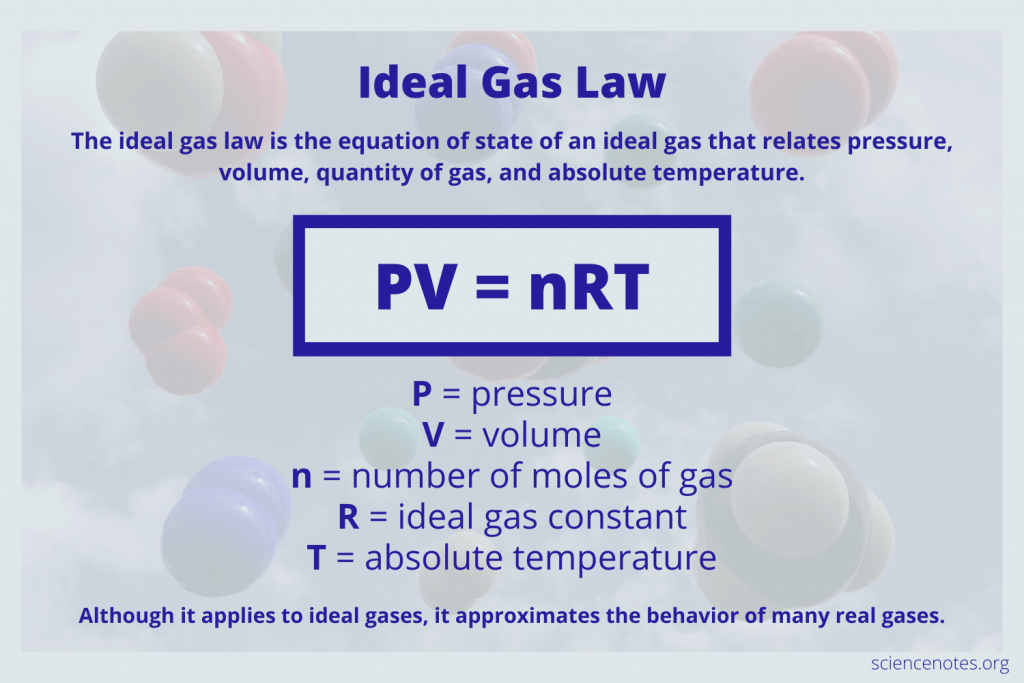
Moving beyond individual gas laws, we have the Ideal Gas Law, which combines the relationships described by Boyle, Charles, and Avogadro into one equation:
PV = nRT
Where:
- P is pressure
- V is volume
- n is the number of moles of gas
- R is the gas constant
- T is the temperature (in Kelvin)
🌡️ Note: The Ideal Gas Law assumes gases are ideal, meaning there are no intermolecular forces and the gas particles occupy zero volume.
Real Gas Laws

While the ideal gas law works well under many conditions, real gases deviate from ideal behavior due to:
- Intermolecular forces between gas molecules.
- The actual volume occupied by gas molecules.
To account for these deviations, chemists use equations like the van der Waals equation:
[P + a(n/V)^2](V - nb) = nRT
Here:
- a accounts for the intermolecular attraction between particles.
- b corrects for the volume occupied by the gas molecules themselves.
Applications of Gas Laws in Everyday Life

Understanding gas laws isn't just academic; it has numerous practical applications:
- Scuba Diving: Gas laws help divers manage pressure changes and avoid decompression sickness.
- Automotive Engines: The air-fuel mixture is governed by gas laws to optimize combustion efficiency.
- Meteorology: Weather patterns and atmospheric pressure are analyzed using gas laws.
- Pharmaceutical Manufacturing: Drug synthesis and packaging often involve manipulating gases under different pressures and temperatures.
These laws help us in:
- Understanding how respiratory systems work by applying principles like Henry's Law for gas solubility.
- Explaining why hot air balloons rise, using Charles's Law.
- Describing how aerosol cans function, based on pressure, volume, and temperature interactions.
Tips for Mastering Gas Laws

Here are some tips to effectively learn and apply gas laws:
- Use Visual Aids: Diagrams and graphs help in visualizing how gas laws work.
- Practice Problems: There's no better way to understand these laws than by applying them to numerical problems.
- Understand the Concepts: Rather than memorizing, understand why these laws work.
- Real-World Examples: Relate gas laws to everyday life for better retention and understanding.
📚 Note: Always keep a periodic table handy; it provides essential values like molar mass, which you'll often need in gas calculations.
Reflecting on gas laws, we've seen how they not only help us understand the physical behavior of gases but also form the backbone of many technologies and natural processes. Gas laws are integral to modern chemistry, physics, and numerous industrial applications. From predicting the behavior of gases in closed systems to helping develop life-saving medical technologies, the knowledge of these laws can be quite empowering. By mastering these concepts, you're not only equipped to excel in your academics but also prepared to tackle real-world problems with a solid scientific foundation.
What’s the difference between real and ideal gases?

+
An ideal gas follows the Ideal Gas Law perfectly, assuming no molecular volume and no intermolecular forces. Real gases, however, deviate from this behavior due to actual molecular volume and attractive forces between particles. The van der Waals equation is one way chemists account for these real-world deviations.
How do gas laws apply to scuba diving?

+
Scuba diving involves understanding how the gas laws relate to changes in pressure with depth. Boyle’s Law is particularly important; as divers descend, the increased pressure decreases the volume of air in their lungs, requiring them to inhale air at higher pressure. Failure to understand these principles can lead to pressure-related injuries.
Can gas laws explain weather patterns?

+
Yes, gas laws are used extensively in meteorology. For instance, air masses move in response to differences in temperature and pressure, as described by the Ideal Gas Law. The relationship between temperature, pressure, and volume helps meteorologists predict weather changes and atmospheric behavior.
