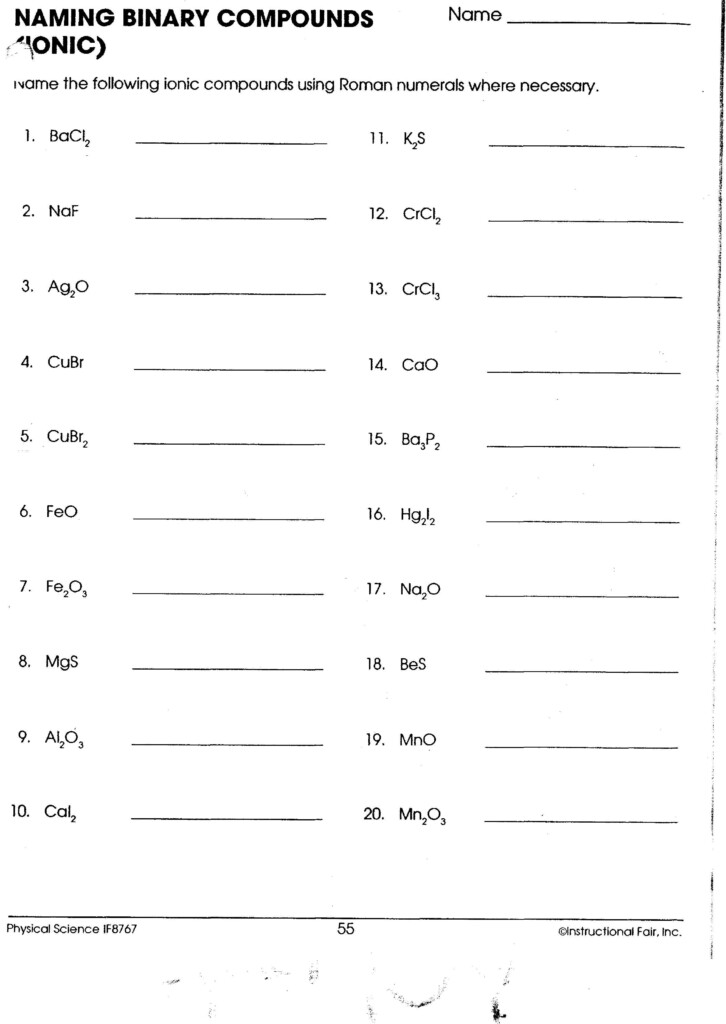
Ionic Compounds Formulas Worksheet: Master the Basics
Are you struggling to master the basics of ionic compounds formulas? Don't worry, you're not alone! Chemistry can seem daunting with its myriad of elements and complex formulas. However, understanding how to write and name ionic compounds is a fundamental skill for anyone studying chemistry. In this detailed guide, we will explore everything you need to know about ionic compounds, from their basic structure to how you can accurately write their formulas. Let's dive in!
What Are Ionic Compounds?

Ionic compounds are types of compounds where the element's atoms are bonded together by the transfer of electrons. This process results in the formation of ions with opposite charges that attract each other, forming an ionic bond. The metal atoms typically lose electrons to become positively charged cations, while non-metal atoms gain these electrons to become negatively charged anions. Here's a simple breakdown:
- Cations: Positively charged ions, usually formed from metals.
- Anions: Negatively charged ions, often formed from non-metals.
⚗️ Note: Ionic bonds involve complete transfer of electrons, unlike covalent bonds which involve sharing of electrons.
How to Write Formulas for Ionic Compounds?

Writing ionic compound formulas isn't as complex as it might seem. Here's a step-by-step guide:
1. Identify the Ions

- Determine the charge of each ion involved. For example, sodium (Na) forms a +1 cation (Na⁺), while chloride (Cl) forms a -1 anion (Cl⁻).
- Use the periodic table or a list of common ions to find the charges if they are not immediately known.
2. Cross-Over Charges

- The charge of one ion becomes the subscript for the other ion. For sodium chloride, Na(1+) and Cl(1-); the subscript 1 is generally omitted, so it’s simply NaCl.
- If the charges are not equal, you’ll need to balance them. For example, calcium (Ca²⁺) and chloride (Cl⁻) will form CaCl₂ because the +2 charge of calcium neutralizes the -1 charge of two chloride ions.
3. Use Parentheses When Necessary
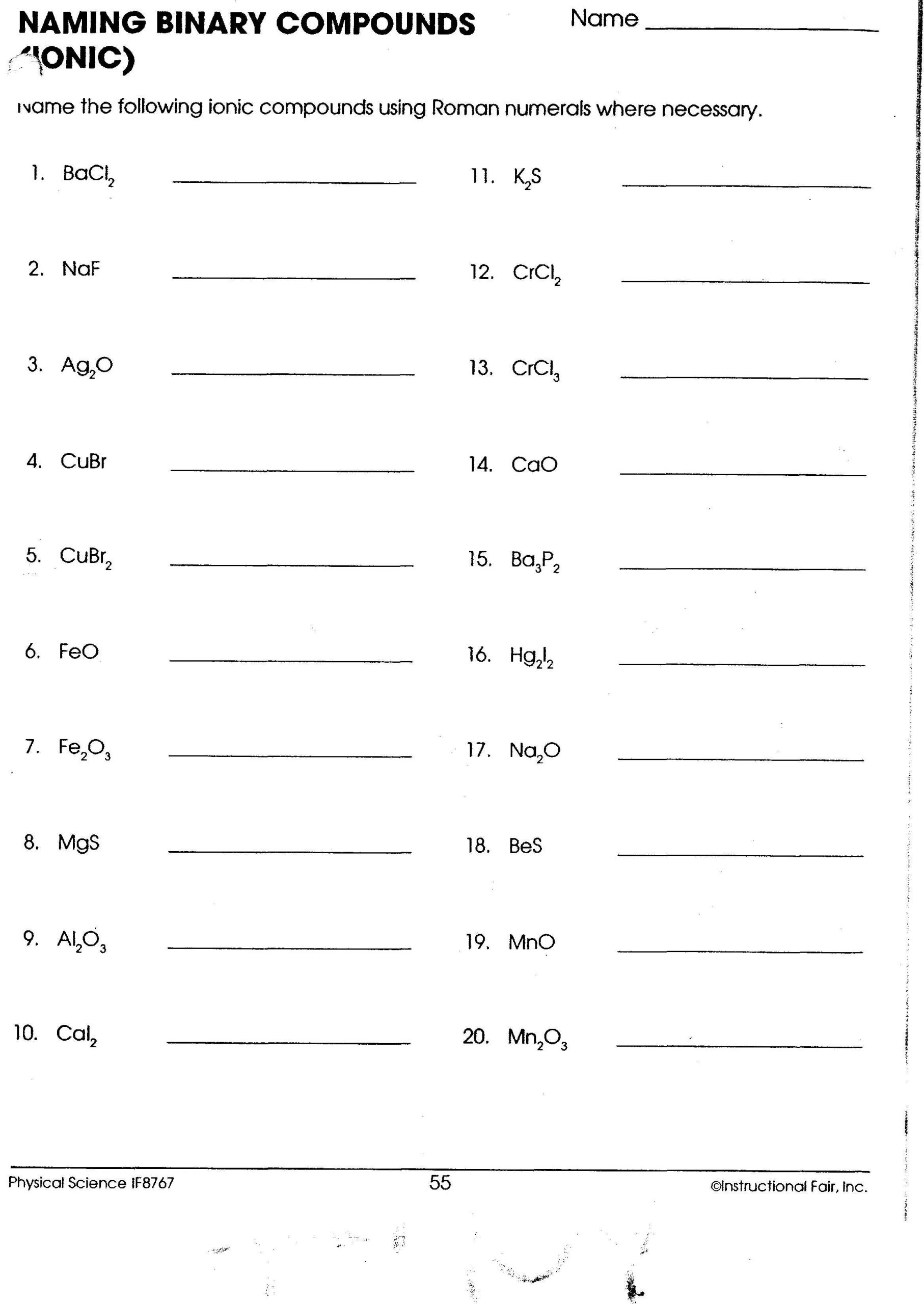
- When a polyatomic ion (an ion made up of more than one atom) needs to be multiplied, use parentheses. For example, sodium sulfate would be Na₂(SO₄).
4. Verify the Formula

- Ensure the charges of the ions sum to zero, maintaining overall neutrality of the compound.
⚗️ Note: The Criss-Cross rule can help simplify the formula writing process, but always verify your formulas for balance.
Naming Ionic Compounds

Naming ionic compounds follows certain conventions to ensure clarity in communication:
Simple Cations and Anions

- Name the cation first followed by the anion. For simple ions, the name of the metal is followed by the name of the anion with its suffix changed to “-ide”. (e.g., Sodium chloride for NaCl).
Transition Metals and Variable Charges

- When a metal can form multiple ions (like iron, which can be Fe²⁺ or Fe³⁺), the Roman numeral system is used to indicate the charge of the metal in the compound (e.g., Iron(II) oxide for FeO or Iron(III) oxide for Fe₂O₃).
Polyatomic Ions

- Polyatomic ions retain their names in the compound. For example, Ammonium nitrate for NH₄NO₃.
⚗️ Note: Always remember that correct naming is critical for clear communication in scientific contexts.
Common Mistakes and How to Avoid Them

- Overlooking Charges: Always verify the charges of the ions to balance the formula correctly.
- Incorrect Use of Subscripts: Subscripts should be used correctly to show the number of atoms or ions needed to balance the charges.
- Failure to Use Parentheses: When dealing with polyatomic ions, parentheses are crucial to indicate multiplication without altering the ion itself.
- Misidentification of Ions: Ensure you're aware of the common charges of ions, especially transition metals which can have variable charges.
Additional Tips for Mastery

Mastery of ionic compound formulas requires practice and understanding:
- Create flashcards for common cations and anions. This helps in memorizing ions and their charges.
- Practice balancing charges until it becomes second nature. Use quizzes or online tools to test your knowledge.
- Understand the concept of oxidation states for elements that can form multiple ions.
⚗️ Note: Regular practice will make the balancing process intuitive and lessen the chance of errors.
What is the difference between a cation and an anion?

+
A cation is a positively charged ion formed when an atom loses one or more electrons. An anion is a negatively charged ion formed when an atom gains one or more electrons.
Why do ionic compounds have high melting points?

+
Ionic compounds have high melting points due to the strong electrostatic forces of attraction between the oppositely charged ions, known as ionic bonds. These forces require significant energy to break, resulting in high melting points.
How can I memorize common polyatomic ions?

+
Creating flashcards with the name, formula, and charge of each ion can be helpful. Also, you can associate the endings like -ate or -ite with the number of oxygen atoms in the ion, making it easier to remember.
Throughout this exploration of ionic compound formulas, we’ve covered the basics from understanding what ionic compounds are, how to write their formulas, to common mistakes and additional tips for mastering the subject. Remember, the key to proficiency in chemistry is not just memorization but understanding the underlying principles and practicing regularly. By applying these guidelines, you’ll soon find yourself confidently handling ionic compounds in your chemistry studies!