Energy Wavelength Frequency: Answer Key Revealed

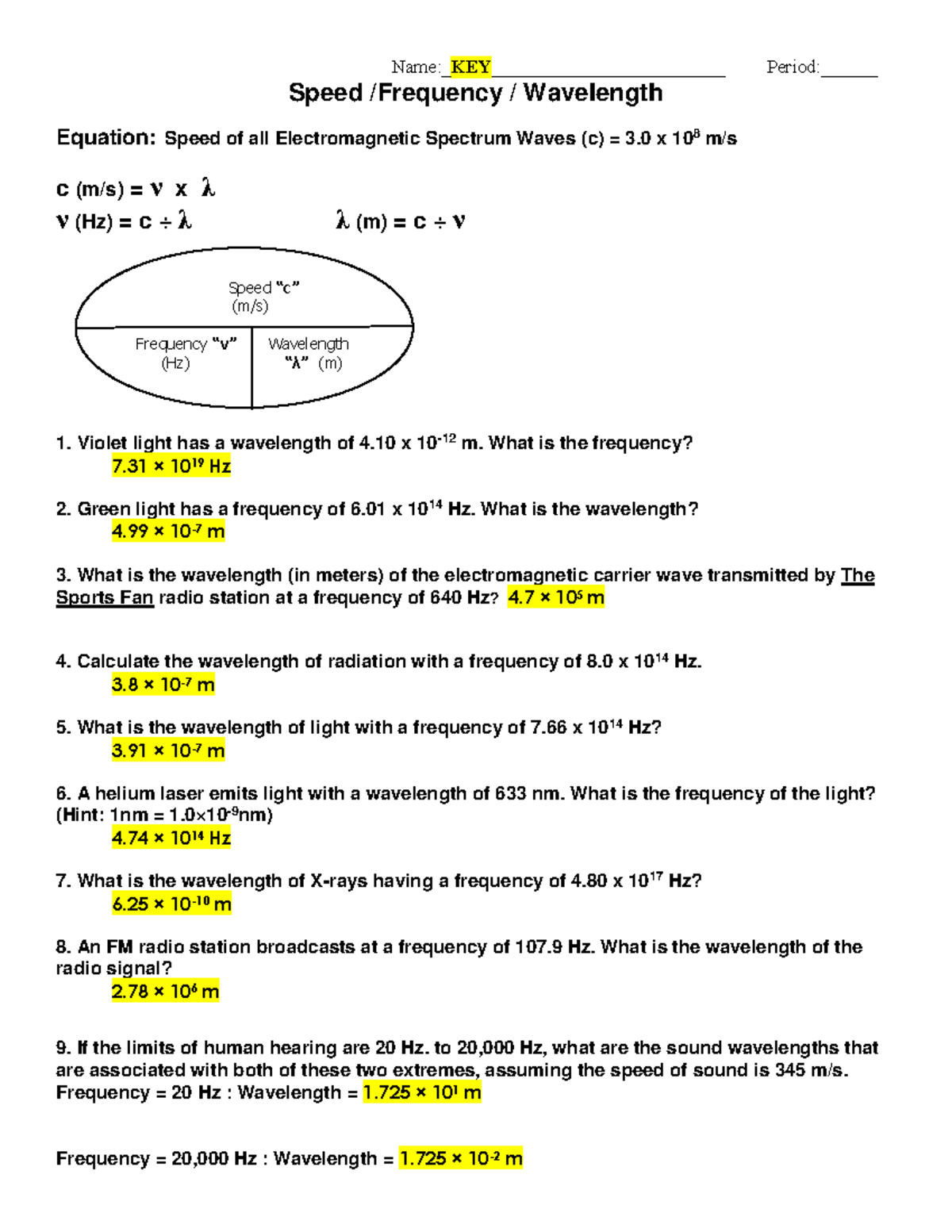
Understanding energy wavelength and frequency forms the cornerstone of various scientific fields, from physics and chemistry to telecommunications and quantum mechanics. This exploration dives into the fascinating world where energy, light, and matter intersect, offering insights into the answer key of how different energies behave, how they can be manipulated, and their practical applications.
Introduction to Energy, Wavelength, and Frequency
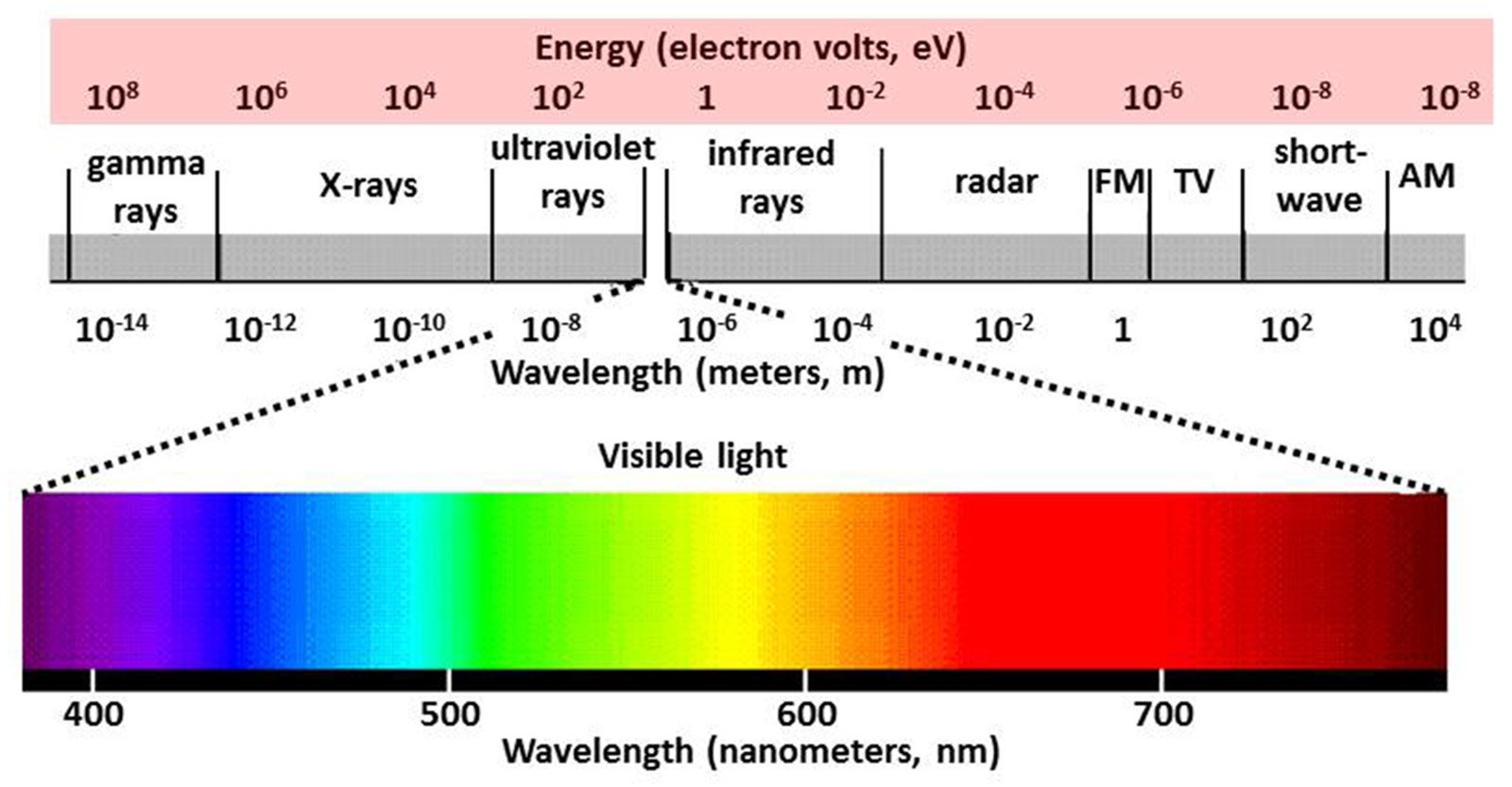

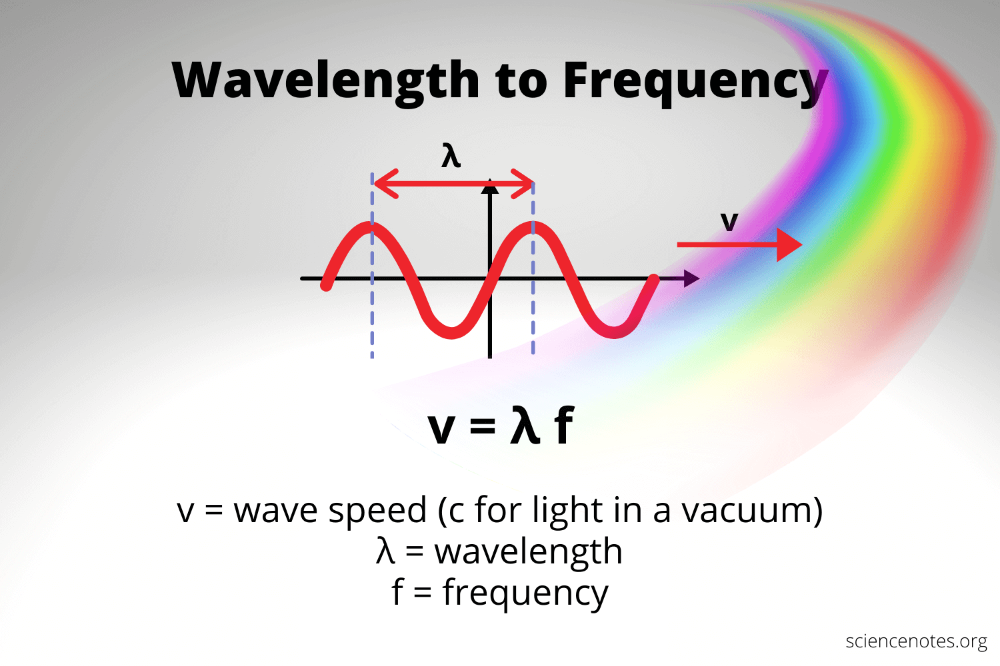
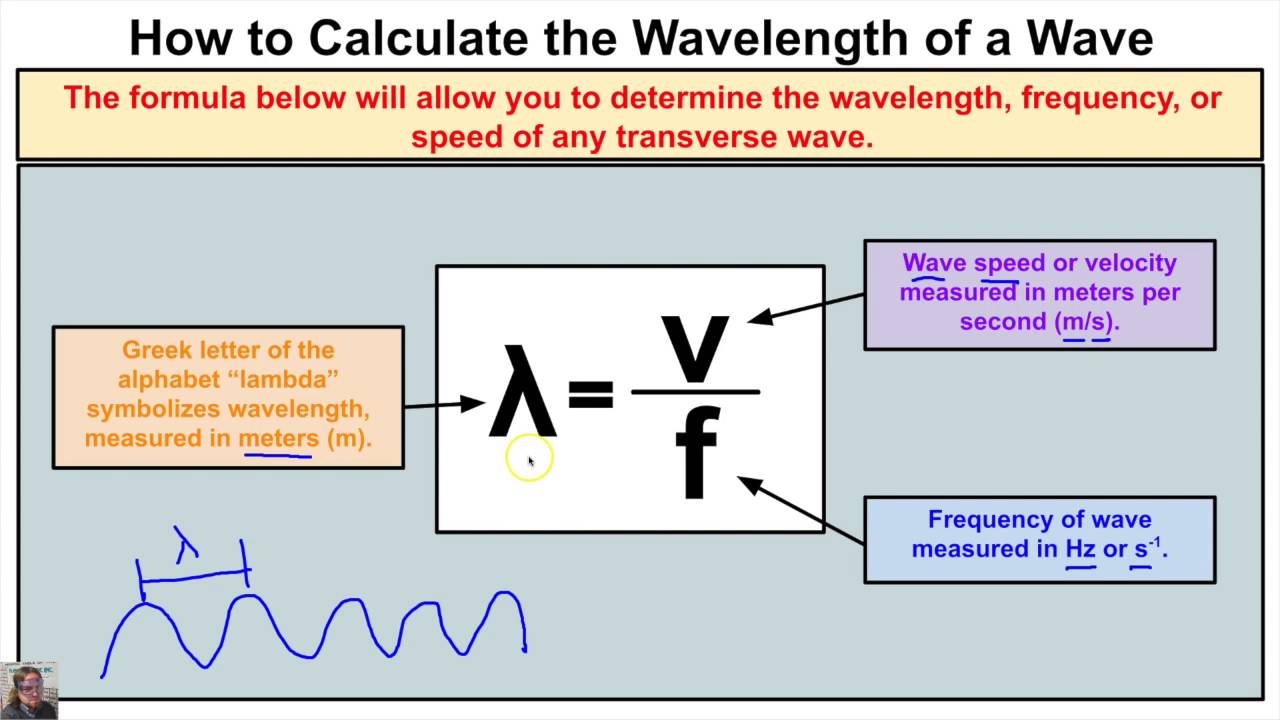
Energy in the physical world often manifests through light, which travels in waves. These waves are described by their wavelength, the distance between two consecutive points in a wave’s cycle, and frequency, the number of cycles that pass a given point per second.
- Wavelength (λ) - measured in meters (m) or nanometers (nm).
- Frequency (f) - measured in Hertz (Hz).
The relationship between these properties and energy is defined by Planck's Equation:
E = h * f
Where:
- E is the energy (in Joules).
- h is Planck’s constant (6.626 × 10-34 J s).
- f is the frequency of the light.
This equation reveals that the energy of a photon is directly proportional to its frequency and inversely related to its wavelength. Higher frequency means higher energy, and shorter wavelength implies higher energy too.
The Spectrum of Light

Light doesn’t exist in isolation but spans across a spectrum, each segment differing in wavelength, frequency, and thus energy. Here’s a breakdown:
Ultraviolet (UV) Radiation

- Wavelength range: 10 nm to 400 nm
- Frequency range: 7.5 × 1015 Hz to 3 × 1016 Hz
- High energy, capable of ionizing atoms, used in UV lamps, sterilization, and tanning.
Visible Light
Visible to the human eye, this spectrum includes:
| Color | Wavelength Range (nm) | Frequency Range (THz) |
|---|---|---|
| Red | 620 - 740 | 400 - 484 |
| Orange | 590 - 620 | 484 - 508 |
| Yellow | 570 - 590 | 508 - 526 |
| Green | 500 - 570 | 526 - 600 |
| Blue | 450 - 500 | 600 - 668 |
| Violet | 380 - 450 | 668 - 789 |
💡 Note: The exact wavelengths for each color can slightly vary between sources, but these values represent the typical ranges.
Infrared (IR) Radiation

- Wavelength range: 700 nm to 1 mm
- Frequency range: 300 GHz to 430 THz
- Lower energy than visible light, involved in thermal radiation and heating.
The Role of Frequency and Wavelength in Technology
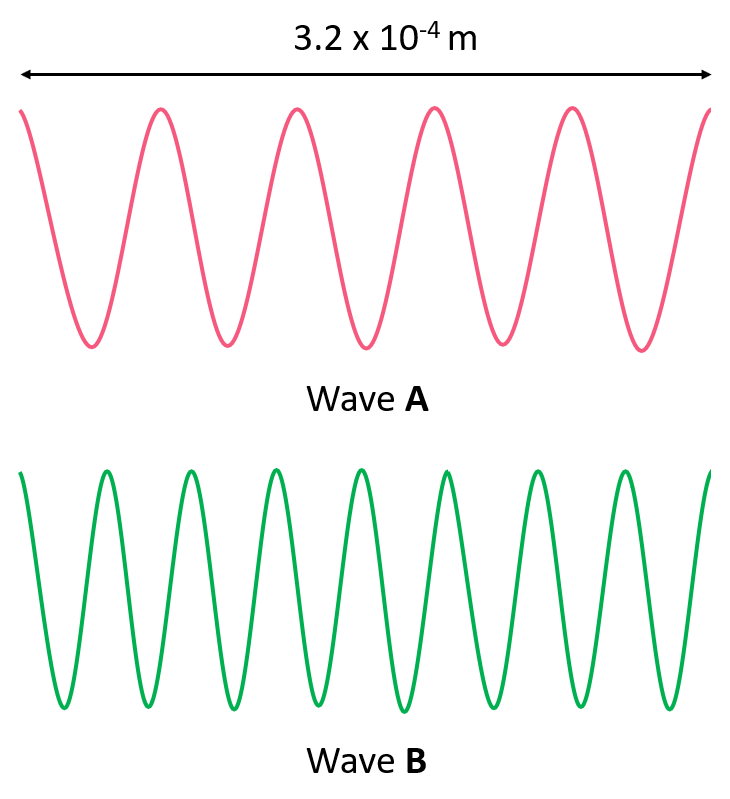
Frequency and wavelength are not just academic interests but are pivotal in shaping modern technology:
Radio Waves

- Frequency: 3 kHz to 300 GHz
- Uses: Broadcasting, wireless communication, radar, navigation.
Microwaves

- Frequency: 300 MHz to 300 GHz
- Uses: Microwave ovens, mobile communications, satellite navigation.
Visible Light

Visible light technology ranges from light-emitting diodes (LEDs) for energy-efficient lighting, laser technology for precision surgery and manufacturing, to photography where different wavelengths are used for artistic and technical photography.
Understanding how energy, frequency, and wavelength interact allows us to innovate:
- Advancements in solar energy capture through understanding light absorption.
- Developments in quantum computing, where energy levels are manipulated at the atomic scale.
- Improving communication technologies by utilizing different parts of the electromagnetic spectrum.
The seamless integration of these principles into daily life and technology showcases the essential nature of mastering energy wavelength frequency for modern advancements.
Applications in Various Fields

The interplay between energy, wavelength, and frequency extends far beyond basic physics, affecting:
Medicine and Healthcare

Medical applications include:
- Using ultraviolet light for sterilization.
- Imaging with X-rays, which are high energy, short wavelength electromagnetic waves.
- Photodynamic therapy, where light of a specific wavelength is used to activate drugs that kill cancer cells.
Communications

In communications:
- Fiber optic cables transmit data using the properties of light.
- Radio and TV broadcasting rely on the different frequencies for signal propagation.
Manufacturing

In manufacturing:
- Lasers with precise wavelengths are used for cutting, welding, and engraving.
Each application illustrates how a deep understanding of energy, wavelength, and frequency can unlock new possibilities and improve efficiency in diverse sectors.
Understanding Quantum Mechanics

The exploration of energy and light waves leads us to quantum mechanics, where the answer key to understanding how energy behaves at the smallest scales becomes evident:
Quantum Energy Levels

Quantum mechanics introduces the concept of discrete energy levels, or quanta. Electrons in atoms can only exist in certain energy states, and the transitions between these states involve absorbing or emitting photons:
- Higher energy transitions (like from n=2 to n=1 in the hydrogen atom) result in the emission of photons with shorter wavelengths (higher energy).
- Lower energy transitions emit photons with longer wavelengths.
This quantum behavior underpins:
- Spectral lines in atomic spectra.
- The operation of lasers where photons of specific wavelengths stimulate electron transitions.
Wave-Particle Duality
Light exhibits both wave-like and particle-like properties:
- Interference patterns in double-slit experiments reveal the wave nature.
- The photoelectric effect shows photons acting like particles with discrete energy packets.
🔬 Note: Wave-particle duality is a cornerstone concept in quantum mechanics that challenges classical physics' macroscopic rules when applied to microscopic phenomena.
Practical Implications and Innovations
Harnessing the principles of energy, wavelength, and frequency leads to real-world applications:
Solar Energy
Photovoltaic cells:
- Convert visible light to electricity using the photoelectric effect.
- Research focuses on optimizing the absorption of different parts of the light spectrum for higher efficiency.
Optical Computing
The development of optical computing:
- Utilizes light’s ability to carry information at high speeds.
- Leverages frequency shifts for data processing and manipulation.
Space Technology
In space:
- Infrared telescopes detect heat signatures in cosmic bodies, revealing processes not visible in the optical range.
- Space exploration uses frequency analysis to understand the composition of celestial objects.
The ability to manipulate and understand the electromagnetic spectrum has expanded our capabilities in every field, from energy harvesting to communication.
In summary, mastering the answer key of energy wavelength frequency unlocks new realms in science and technology. From the foundation laid by Planck's equation, through the colorful spectrum of visible light, to the cutting-edge applications in quantum computing and beyond, the intricacies of light and energy are vital to our progress. As we continue to push the boundaries of this knowledge, we pave the way for innovations that enhance our understanding of the universe and improve our quality of life.
What is the difference between wavelength and frequency?
+Wavelength is the distance between two consecutive points in a wave’s cycle, typically measured in meters or nanometers, while frequency is the number of cycles that pass a given point per second, measured in Hertz (Hz). They are inversely related; as frequency increases, wavelength decreases, and vice versa.
How does understanding energy, wavelength, and frequency impact daily technology?
+It impacts everything from the way we communicate (wireless signals, radio waves), to how we see light (visible spectrum), to medical treatments (radiation therapies) and more. Technologies like LEDs, lasers, and even solar cells depend on these principles to function efficiently.
Why is the photoelectric effect important in understanding energy and light?
+The photoelectric effect proved that light can behave as both a wave and a particle. This quantum behavior is crucial for technologies like solar cells, where photons transfer their energy to electrons, allowing the conversion of light into electricity.
Can different wavelengths of light affect our health?
+Yes, UV light can cause sunburns and increase the risk of skin cancer, whereas visible light impacts our circadian rhythms. Infrared can be beneficial in thermal therapies, but overexposure can also lead to heat-related issues.
How is wavelength used in medical diagnostics?
+Wavelength-specific imaging like X-rays or MRI uses different wavelengths to penetrate tissues at varying depths, providing doctors with insights into the internal structures of the body for diagnosis and treatment planning.