Unlock Answers: Empirical & Molecular Formula Worksheet
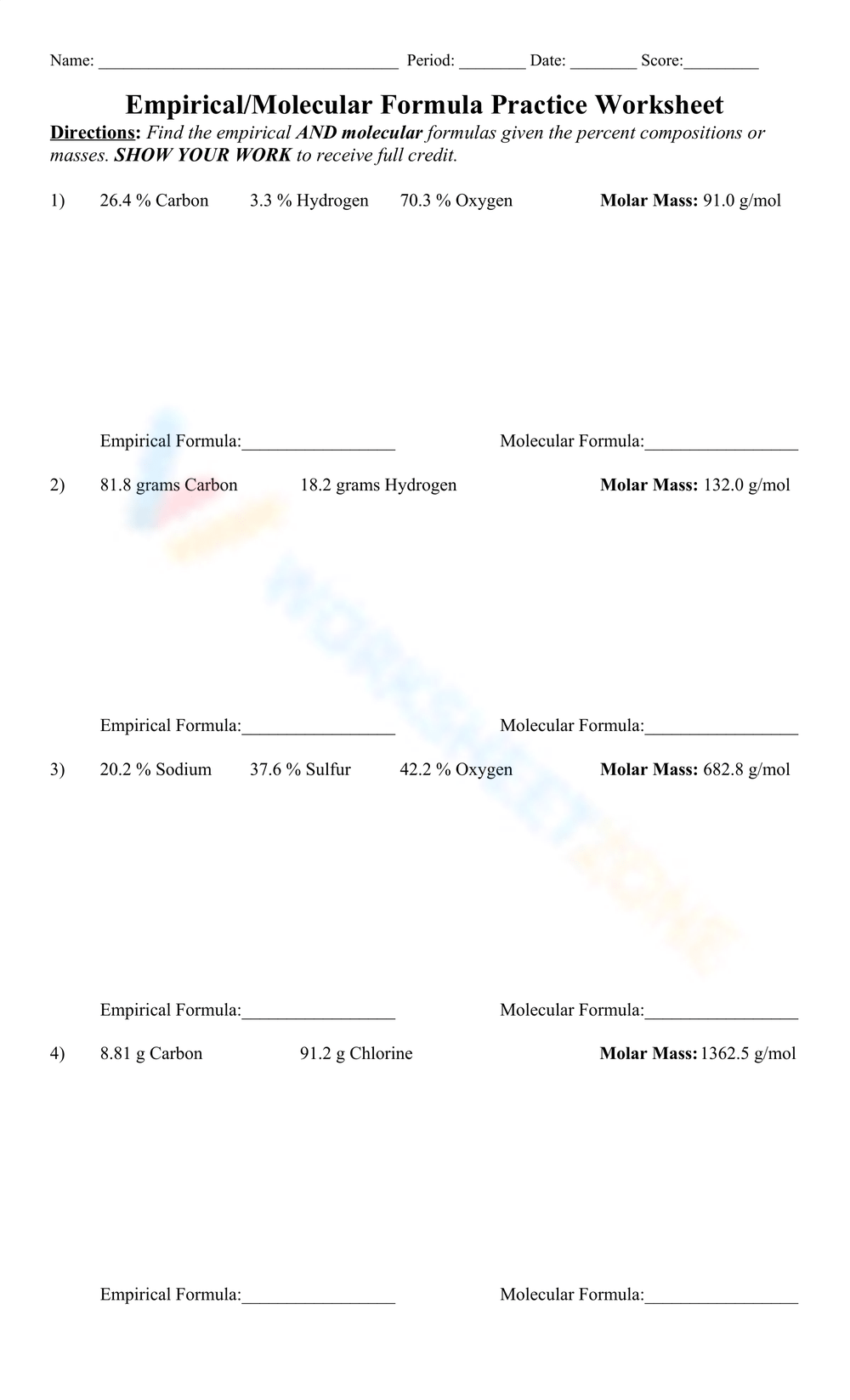
Worksheets that focus on empirical and molecular formulas are fundamental tools for students to grasp the intricacies of chemical composition. Empirical formula is the simplest whole-number ratio of atoms present in a compound. On the other hand, the molecular formula tells us the actual number of atoms of each element in one molecule of the compound. Both formulas are critical in chemistry, aiding in understanding compound behavior and synthesis.
Understanding Empirical and Molecular Formulas

To understand these formulas better, let's look into the definitions and importance:
- Empirical Formula: Derived from experimentation, this formula represents the simplest whole-number ratio of elements in a compound. For example, glucose has the molecular formula C6H12O6, but its empirical formula is CH2O.
- Molecular Formula: This is the actual ratio of the atoms of each element in a molecule, often a multiple of the empirical formula. For instance, in the case of glucose, the molecular formula (C6H12O6) is six times its empirical formula.
Steps to Calculate Empirical Formula

Here’s a step-by-step guide on how to determine an empirical formula:
- Convert Mass to Moles: Use atomic masses to convert the given mass of each element into moles.
- Find the Mole Ratio: Divide each mole value by the smallest mole value calculated in the previous step to get the simplest whole number ratio.
- Empirical Formula: Write the formula using the ratios found.
📝 Note: If ratios aren't whole numbers, you can multiply through by the smallest integer that converts them to whole numbers.
Steps to Calculate Molecular Formula

Once you have the empirical formula, the following steps help you calculate the molecular formula:
- Determine Molar Mass: Find the molecular mass of the compound, which can be given or you might need to calculate it using different methods.
- Compare Empirical and Molecular Mass: Divide the molecular mass by the mass of the empirical formula to find a multiplier.
- Calculate Molecular Formula: Multiply each element in the empirical formula by the multiplier to get the molecular formula.
| Compound | Empirical Formula | Molecular Formula | Molecular Mass |
|---|---|---|---|
| Glucose | CH2O | C6H12O6 | 180.16 g/mol |
| Ethane | CH3 | C2H6 | 30.07 g/mol |

Practical Applications

Understanding these formulas has practical applications in:
- Pharmacology for drug synthesis
- Analytical chemistry for identifying unknown substances
- Material science for new material development
🔬 Note: In industrial applications, knowing the molecular structure can influence the properties of the material, thus affecting its use in various technologies.
To wrap up, mastering empirical and molecular formulas not only deepens one's understanding of chemistry but also has far-reaching implications in various scientific and industrial fields. By following the steps outlined above, students can confidently tackle problems in chemistry worksheets and beyond. This knowledge allows one to decode the complex language of molecules and atoms, bridging theoretical chemistry with practical applications. Now you're better prepared to navigate the exciting world of chemical formulas with a solid foundation, setting the stage for further exploration in chemistry.
What’s the difference between empirical and molecular formulas?

+
The empirical formula gives the simplest whole-number ratio of atoms in a compound, while the molecular formula gives the actual number of atoms in one molecule of the compound.
Why don’t we always use the molecular formula?

+
Using the empirical formula is often sufficient for many calculations and in understanding the basic structure of a compound, especially when the molecular formula isn’t known or doesn’t matter for the context.
How can empirical formulas change with different isotopes?

+
Isotopes don’t change the empirical formula since it’s based on the ratio of atoms, not the mass. However, the isotopic composition might affect the molecular mass.