5 Ways to Distinguish Elements, Mixtures, and Compounds in Worksheets

In the realm of chemistry education, distinguishing between elements, mixtures, and compounds is crucial for understanding chemical reactions and the nature of matter. Students often find this differentiation challenging, but with the right approaches, it becomes more manageable. Here are five effective strategies to help students distinguish these concepts within worksheets and beyond:
1. Visual Representation
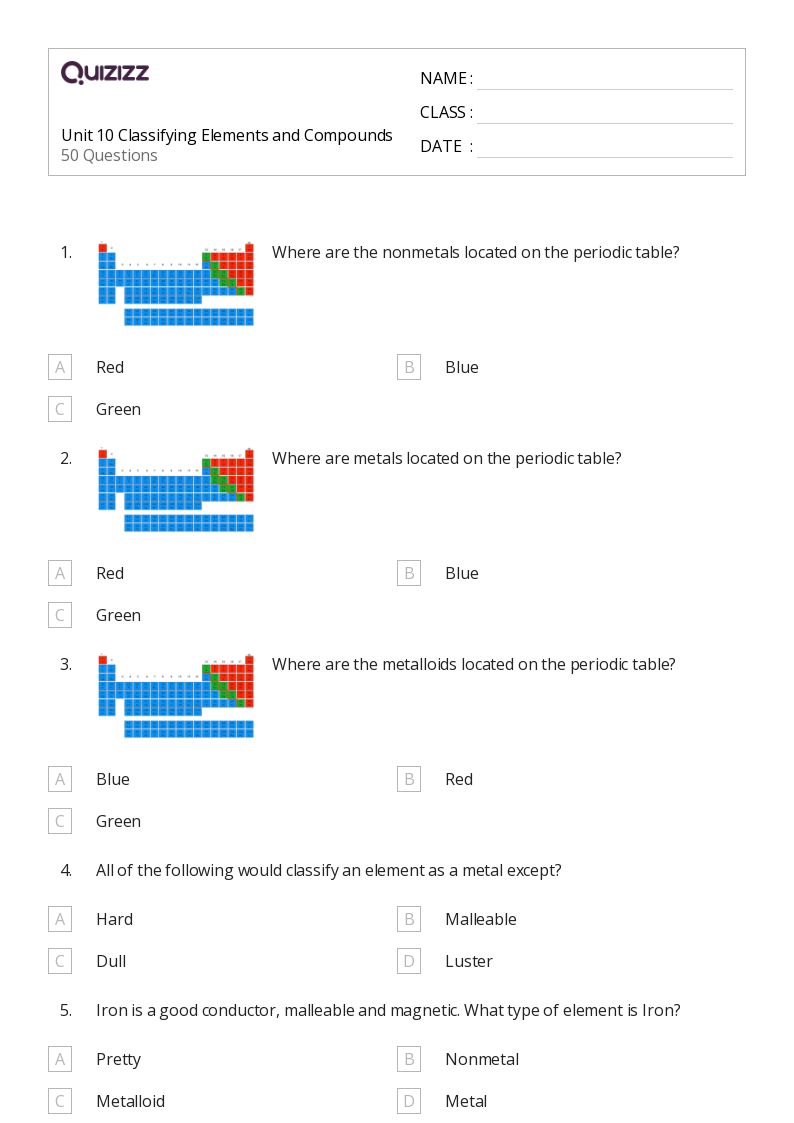
Visual aids are powerful educational tools. Using diagrams, charts, or illustrations helps in clarifying abstract concepts:
- Elements: Represented by simple atomic symbols or color-coded images to highlight their unique atomic structure.
- Mixtures: Use overlapping circles or Venn diagrams to show how components retain their identity but mix together.
- Compounds: Illustrate them with bonded atoms in a fixed ratio, using different colors or bonding lines to demonstrate molecular structure.

2. Concept Check through Examples

Providing examples alongside definitions can solidify understanding:
| Category | Example |
|---|---|
| Elements | Hydrogen (H), Oxygen (O), Sodium (Na) |
| Mixtures | Air (O2 + N2 + CO2 + etc.), Saltwater (NaCl + H2O) |
| Compounds | Water (H2O), Carbon Dioxide (CO2) |

🔍 Note: Ensure that students understand that in mixtures, elements and compounds can still be separated by physical means, whereas compounds require chemical reactions for decomposition.
3. Interactive Activities

Interactive learning keeps students engaged and promotes active learning:
- Matching Games: Students can match symbols or names to descriptions of elements, mixtures, or compounds.
- Sorting Tasks: Provide cards with names of different substances and have students categorize them.
- Lab Simulations: Use virtual labs where students can see how mixtures can be separated and compounds formed through reactions.

4. Questioning Techniques

Well-crafted questions encourage critical thinking:
- Can this substance be broken down further into simpler substances? (Elements cannot; compounds can.)
- Is the composition of the substance variable or fixed? (Compounds have a fixed composition; mixtures can vary.)
- Do the components retain their original properties in this substance? (In mixtures, yes; in compounds, properties change upon bonding.)
5. Mnemonics and Memory Aids

Mnemonic devices can aid in memorization and differentiation:
- Elements: “E - Enter alone” - Elements exist alone with unique atomic numbers.
- Mixtures: “M - Mixable” - Can be mixed, and ingredients maintain their identity.
- Compounds: “C - Combine to change” - Combine different elements to form a new substance with different properties.
💡 Note: Encourage students to come up with their mnemonics for better recall.
To effectively differentiate elements, mixtures, and compounds, a multifaceted approach is essential. By integrating visual aids, real-world examples, interactive activities, thought-provoking questions, and memory aids, educators can facilitate a deeper understanding of these fundamental concepts. This not only aids in better comprehension but also helps in preparing students for more complex chemistry topics. By employing these strategies, students can grasp the distinct properties and behaviors of elements, mixtures, and compounds, setting a solid foundation for their further learning in chemistry.
Why is it important to distinguish between elements, mixtures, and compounds?

+
Understanding the difference allows us to predict chemical behavior, understand reactions, and manage substances in various environments effectively.
How can teachers assess students’ understanding of these concepts?

+
Teachers can assess understanding through quizzes, lab experiments where students must identify or create substances, and through discussions where students explain differences.
What are some common mistakes students make when identifying elements, mixtures, and compounds?

+
Students often confuse compounds with mixtures due to not understanding the uniformity of composition or the method of separation involved. They might also mistake elements for compounds if they don’t recognize the atomic number or element’s symbol.