7 Tips for Mastering Electron Configurations Easily

Electron configurations play a crucial role in understanding the behavior and properties of atoms in chemistry. Whether you're a student grappling with the concepts of atomic structure or a professional aiming to master the nuances of quantum mechanics, mastering electron configurations is essential. This article will explore seven tips that can help you easily understand and apply electron configurations in your studies and practical applications.
1. Understand the Basics

Before diving into the complexities, grasp the fundamental concepts:
- Aufbau Principle: Electrons fill atomic orbitals in order of increasing energy.
- Pauli Exclusion Principle: No two electrons in an atom can have the same four quantum numbers.
- Hund’s Rule: Electrons occupy each available orbital singly before pairing up with electrons of opposite spins.
Understanding these principles forms the bedrock of electron configurations. Familiarize yourself with the periodic table, as its structure directly reflects electron distribution.
2. Use the Periodic Table as a Reference
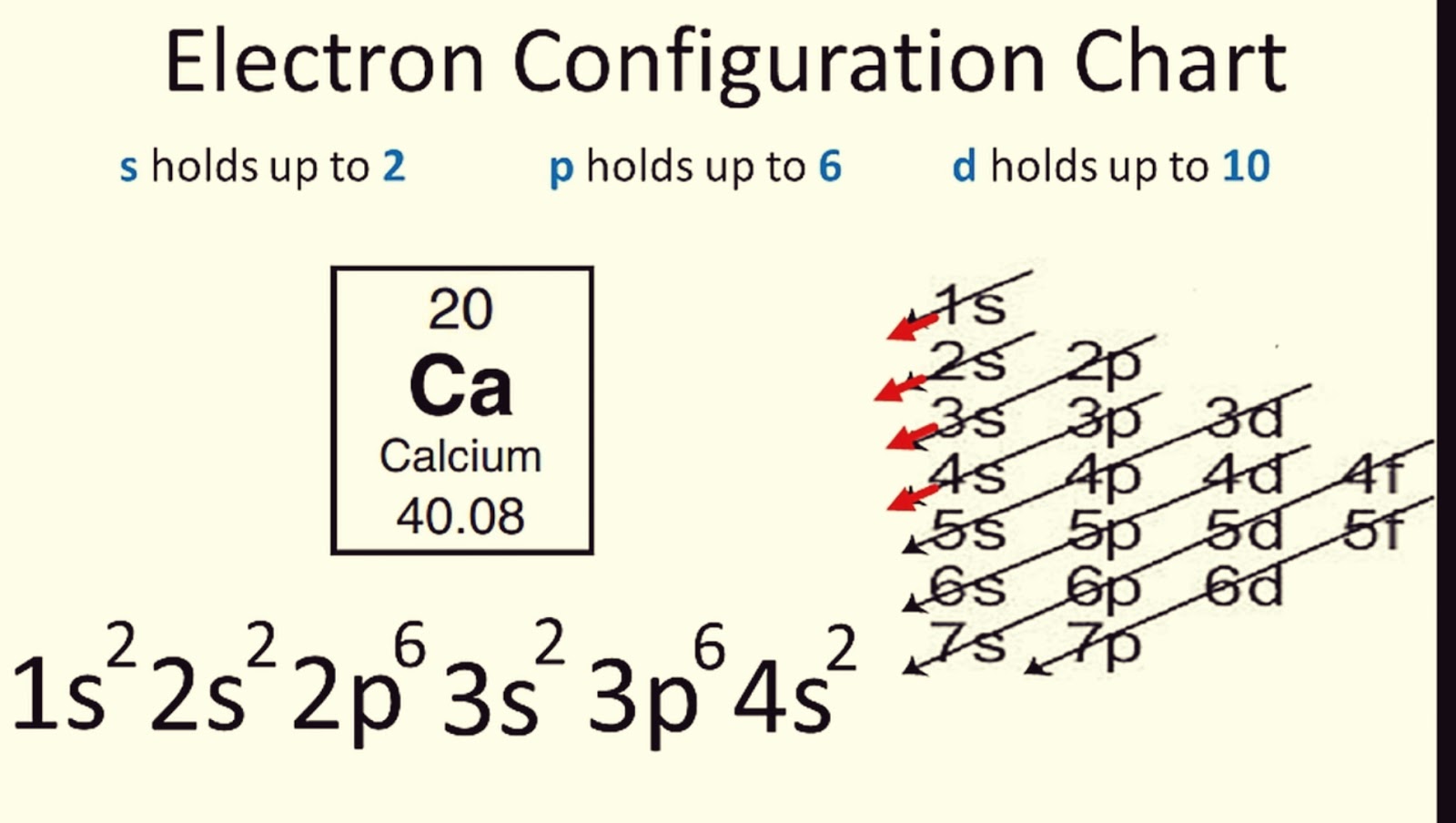
The periodic table isn’t just a chart; it’s a tool for electron configurations:
- The blocks (s, p, d, f) indicate where electrons are added.
- Each row (period) corresponds to the energy level (n).
- The columns (groups) tell you the number of valence electrons.
🔍 Note: Always remember that for transition metals (d-block), the Aufbau principle requires a slight modification where you fill the 4s before the 3d orbitals.
3. Master the Orbital Filling Order

The filling order of atomic orbitals can seem arbitrary but follows a pattern:
| Energy Level | Sublevel | Order |
|---|---|---|
| 1 | s | 1 |
| 2 | s | 2 |
| 2 | p | 3 |
| 3 | s | 4 |
| 3 | p | 5 |
| 4 | s | 6 |
| 3 | d | 7 |

This pattern, known as the Aufbau order, can be derived from the diagonal rule or the Aufbau principle. Practice by writing out electron configurations for several elements.
4. Practice with Noble Gas Shortcuts

Noble gas shorthand simplifies electron configurations by using the symbol of the nearest noble gas to represent the core electrons:
- For example, Sodium (Na): [Ne] 3s1
This method not only reduces writing but also helps to quickly identify the valence electrons, which are key in chemical bonding.
5. Learn to Recognize Valence Shell Electron Pairs

Valence shell electrons are crucial for understanding reactivity:
- They are involved in bonding.
- Look for electrons in the outermost shell or the highest s and p orbitals.
Being adept at quickly determining valence shell electron configurations helps in predicting how elements will react with each other.
6. Utilize Visual Aids

Visual representation of electron configurations using diagrams like:
- Orbital diagrams where boxes represent orbitals, and arrows show the electron’s spin.
- Lewis dot structures for showing valence electrons.
These visual aids can make abstract concepts more tangible. Try drawing electron configurations for elements across the periodic table to solidify your understanding.
7. Create Mnemonics and Memory Aids

To remember the order in which orbitals are filled:
- Create a mnemonic phrase for the Aufbau principle, like “1s2, then 2s2, 2p6, 3s2, 3p6, 4s2, 3d10, and so on.”
- Associate shapes of orbitals (s - sphere, p - dumbbell, d - cloverleaf) with their respective filling orders.
Memory aids can turn a cumbersome task into an enjoyable challenge, easing the learning process.
The journey to mastering electron configurations might seem daunting at first, but with these tips, you'll find it much easier to visualize, understand, and apply these principles in chemistry. As you delve deeper into atomic structures, the elegance of electron configurations will reveal itself in the patterns of the periodic table and the behavior of matter. Whether for academic purposes or for professional development, the ability to confidently handle electron configurations can make a significant difference in your grasp of chemistry.
What is the difference between an orbital and an electron shell?

+
An electron shell is a region around the nucleus where electrons with the same principal quantum number (n) reside. Within each shell, there are orbitals, which are regions within a shell where electrons with specific quantum numbers are likely to be found. Each shell can contain multiple orbitals (s, p, d, f), and electrons fill these orbitals according to their energy levels and the principles of electron configuration.
How do electron configurations relate to an atom’s reactivity?
+
Electron configurations directly influence an atom’s reactivity by determining the number of valence electrons. The valence electrons are the outermost electrons involved in chemical bonding. The more unpaired electrons or the fewer electrons needed to complete the outer shell, the more reactive the atom tends to be.
Why do we use the Noble Gas shorthand?

+
The Noble Gas shorthand is used to simplify the notation of electron configurations. By using the symbol of the nearest noble gas, which has a full electron shell, we can represent the core electrons (inner electrons) of an atom without having to write out each electron individually. This shorthand focuses on the valence electrons, which are key to understanding an element’s chemical behavior.