Mastering Easy Balancing Equations with Free Worksheet

Balancing chemical equations is a fundamental skill in chemistry, providing a glimpse into how elements interact in various reactions. When atoms of elements react, they do so in precise ratios to ensure that mass is conserved, leading to balanced equations. This process can seem daunting at first, but with practice and understanding, it becomes second nature. Here’s how you can master the art of balancing chemical equations using free worksheets.
Understanding Chemical Equations

Before diving into balancing, let’s grasp what a chemical equation represents:
- Reactants: Substances that enter into a reaction.
- Products: Substances that are formed as a result of the reaction.
These are represented with an arrow in the middle, pointing from reactants to products:
[ \text{Reactants} \rightarrow \text{Products} ]
The Process of Balancing Chemical Equations

Balancing chemical equations involves adjusting coefficients (the numbers in front of each formula) so that the number of atoms of each element is the same on both sides of the equation. Here are the steps:
- List the elements: Begin by listing each element involved in the equation.
- Count the atoms: Determine the number of atoms of each element on both sides.
- Change coefficients: Adjust the coefficients to balance the number of atoms. Start with elements that appear in only one reactant and one product.
- Recheck: Recount the atoms after changing coefficients to ensure balance.
Let's look at an example:
\[ \text{H}_2 + \text{O}_2 \rightarrow \text{H}_2\text{O} \]
- Hydrogen: 2 on the left, 2 on the right (already balanced).
- Oxygen: 2 on the left, 1 on the right. This needs balancing.
Place a coefficient of 2 in front of H2O:
\[ \text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} \]
Now oxygen is balanced, but we have 4 hydrogen atoms on the right, so:
\[ 2\text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} \]
💡 Note: Remember, you can change coefficients but not the subscripts within chemical formulas, as this changes the compound entirely.
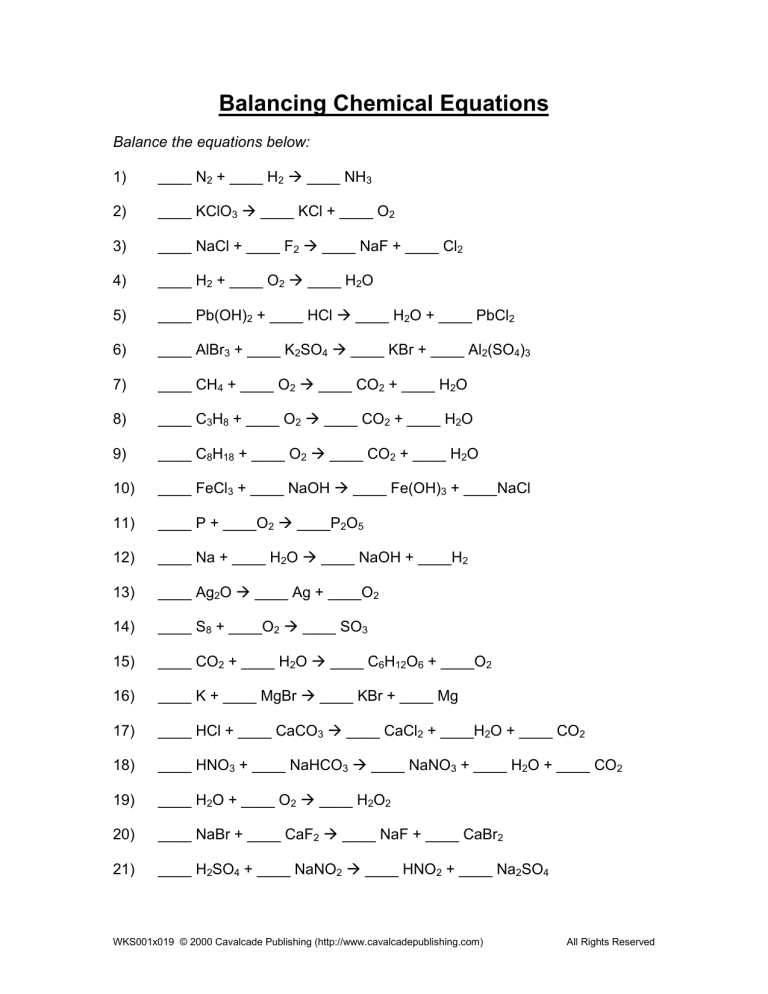
Using Free Worksheets to Practice

Free worksheets are invaluable tools for mastering equation balancing. Here’s how to make the most of them:
- Variety: Worksheets provide a diverse range of equations from simple to complex, allowing for gradual skill progression.
- Feedback: Many worksheets include answers or hints, offering immediate feedback to help learn from mistakes.
- Accessibility: Free worksheets can be found online, making it easy to access and practice at any time.
Common Challenges and Tips

Students often face these challenges when balancing equations:
- Oxidation Numbers: For redox reactions, understanding oxidation states is crucial.
- Fractional Coefficients: Sometimes, fractions might appear in the balancing process, requiring further adjustment.
- Polyatomic Ions: Keep polyatomic ions intact when possible to simplify balancing.
Here are some tips:
- Start with elements that appear in only one reactant and product.
- Balance hydrogen and oxygen last, as they often appear in multiple places.
- Check your work: After balancing, verify that the total charge and the total number of atoms are equal on both sides.
Advanced Techniques

As you become more proficient, consider these advanced techniques:
| Method | Description |
|---|---|
| Algebraic Method | Use equations to solve for coefficients. |
| Inspection Method | Intuitive approach by trial and error. |
| Redox Balancing | Split reactions into oxidation and reduction half-reactions and balance them. |

In summary, mastering the balance of chemical equations involves understanding the underlying concepts, practicing with various methods, and using resources like free worksheets to refine your skills. Remember that balance is not just about numbers; it's about the precision of nature. With time and practice, you'll find this process not only educational but also fascinating, offering a deeper insight into how elements interact in the world around us.
Why is balancing chemical equations important?

+
Balancing chemical equations is crucial to understand how elements interact in reactions, ensuring conservation of mass and charge, which is fundamental to all chemical processes.
What’s the easiest way to balance an equation?

+
The simplest approach often involves the inspection method, starting with elements that appear only once on each side and adjusting coefficients intuitively until balance is achieved.
Can I ever have a balanced equation with fractions?

+
Yes, but it’s usually discouraged. When fractions appear, multiply the entire equation by a common factor to clear them.
What should I do if a polyatomic ion appears in an equation?

+
Keep the polyatomic ion as a whole when balancing to simplify the process. However, ensure that the ion’s count is the same on both sides.
Are there online tools to help balance equations?
+
Yes, various websites offer online equation balancers that can be useful for checking your work or learning different balancing techniques.



