5 Easy Steps to Master Covalent Bond Drawing

Introduction to Covalent Bonds

Covalent bonds are fundamental to the study of chemistry, representing the type of bonds that hold atoms together through the sharing of electrons. Understanding how to draw these bonds correctly is key for anyone learning chemistry, whether you’re a student, an aspiring chemist, or simply someone fascinated by molecular structures. In this detailed blog, we will explore five easy steps to master covalent bond drawing, ensuring that you can confidently construct molecular diagrams that are both accurate and informative.
Step 1: Identify the Atoms Involved

The first step in drawing covalent bonds is to identify the atoms involved in the compound. Each element has a specific electron configuration, with the outermost electrons (valence electrons) participating in bonding:
- Hydrogen - 1 valence electron
- Carbon - 4 valence electrons
- Oxygen - 6 valence electrons
- Nitrogen - 5 valence electrons
- Fluorine - 7 valence electrons
💡 Note: Elements in the same group have the same number of valence electrons, making it easier to identify and draw bonds once you memorize this pattern.
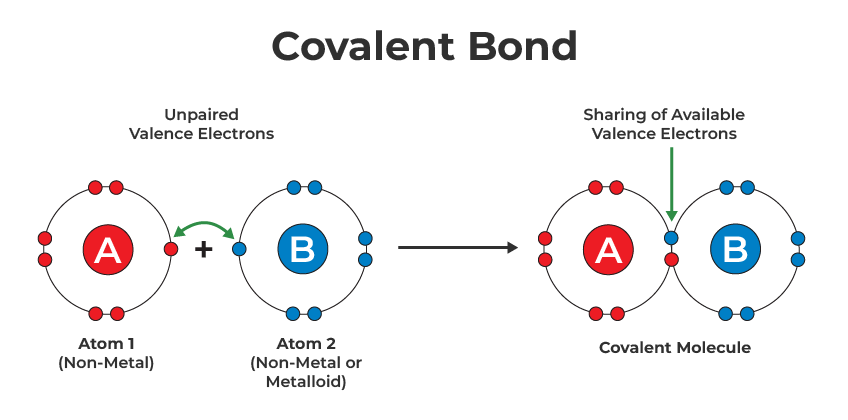
Step 2: Determine Electron Sharing
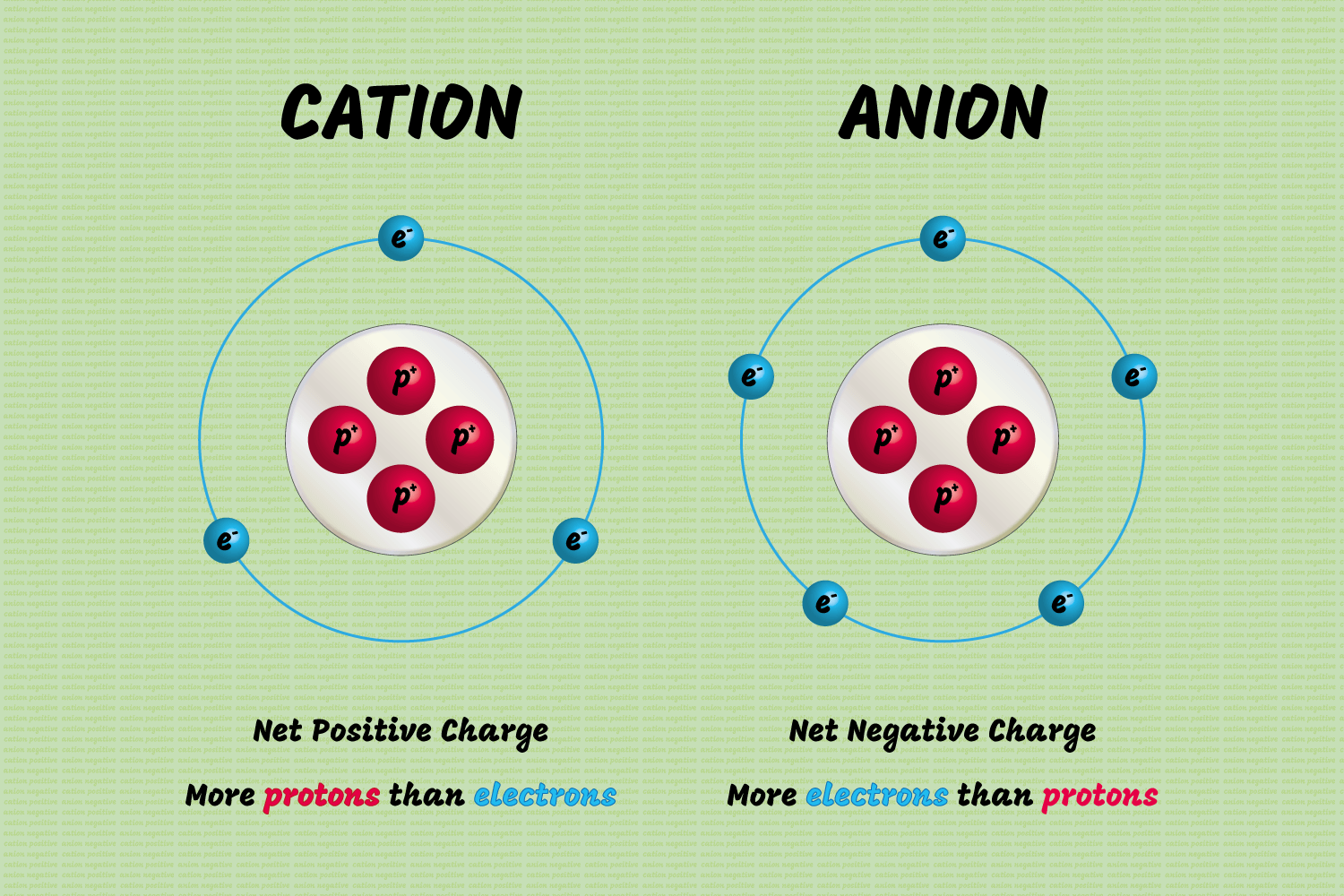
Once you have the atoms, the next step is to determine how many electrons each atom needs to complete its valence shell. Here’s how:
- Hydrogen needs 1 more electron to achieve a duet (2 electrons)
- Carbon needs 4 more electrons for an octet (8 electrons)
- Oxygen needs 2 more electrons for an octet (8 electrons)
- Nitrogen needs 3 more electrons for an octet (8 electrons)
- Fluorine needs 1 more electron for an octet (8 electrons)
The sharing of electrons creates pairs of electrons, which are drawn between atoms.
💡 Note: The goal is to achieve a stable electron configuration, which is why atoms bond. Inert gases, like Helium, do not form bonds because their outermost shell is already full.
Step 3: Apply the Octet Rule

The octet rule states that atoms tend to bond in such a way as to complete their outer electron shells, aiming for 8 electrons (except for Hydrogen and Helium which aim for 2). Here’s how you apply this:
- Arrange the electrons around each atom to achieve this stability.
- Use lines to represent pairs of shared electrons.
Example:

Step 4: Check Formal Charges

After drawing the initial bonds, you should check the formal charge of each atom. Formal charge helps determine if the electron distribution is logical:
- FC = # of valence electrons - # of bonds - nonbonding electrons
If formal charges are too high or too low, you may need to adjust by introducing resonance structures or rechecking the bond distribution.
| Atom | Valence Electrons | # of Bonds | Lone Pairs | Formal Charge |
|---|---|---|---|---|
| C (Carbon) | 4 | 4 | 0 | 0 |
| O (Oxygen) | 6 | 2 | 2 | 0 |
| H (Hydrogen) | 1 | 1 | 0 | 0 |

💡 Note: Formal charges should be as close to zero as possible. Deviations indicate the need for further examination or correction of the structure.
Step 5: Review and Refine

The last step involves reviewing your work:
- Check if all atoms have achieved their desired electron configuration.
- Ensure bond angles are approximately as expected (e.g., 109.5° for tetrahedral carbon).
- Look for any unpaired electrons that might need to be paired through resonance.
Refine any mistakes or reevaluate if you notice something that seems incorrect or unconventional.
Summary
In this comprehensive guide, we’ve explored the five easy steps to master drawing covalent bonds, from identifying participating atoms to reviewing and refining your molecular structures. Drawing covalent bonds is an art that requires understanding electron sharing, octet completion, and the influence of formal charges. By following these steps, you can visualize the fascinating world of chemistry with accuracy and confidence, enhancing your grasp of how matter interacts at the smallest scale.
What is the primary goal of covalent bonding?

+
The primary goal of covalent bonding is to achieve a stable electron configuration, typically an octet of electrons, for each atom involved, allowing them to reach a lower energy state and increase stability.
Why do we use lines to represent covalent bonds?

+
Lines are used because each line represents a pair of electrons that are shared between the bonding atoms. This visual aid simplifies the depiction of chemical bonds in molecular diagrams.
What is the significance of formal charges in covalent bond drawing?

+
Formal charges help in checking whether the distribution of electrons is logical and if the structure drawn is likely the most stable configuration. Deviations from zero indicate potential issues with electron distribution or the need for resonance structures.