Counting Atoms Worksheet Answers: H2O Breakdown
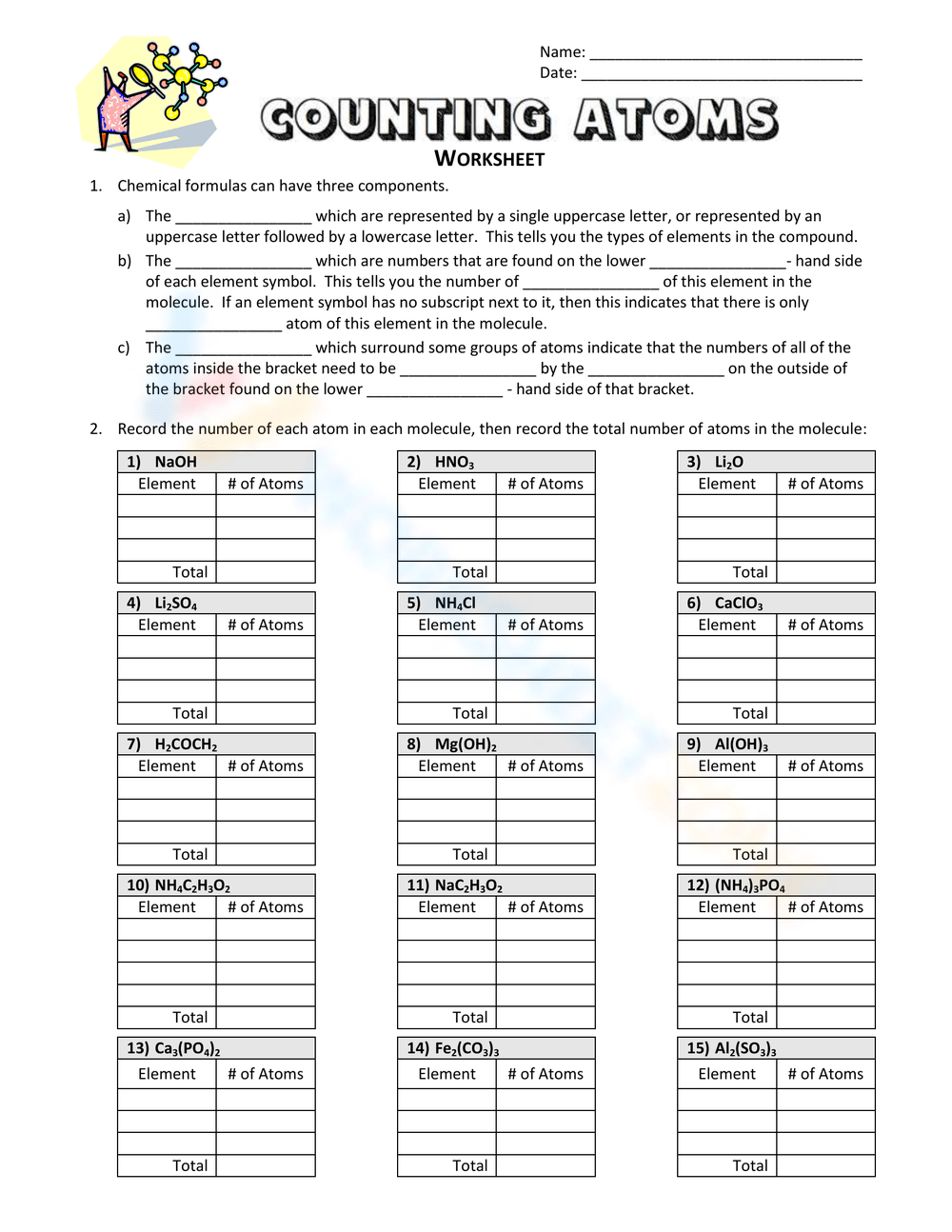
Understanding Chemical Formulas and Counting Atoms: The Example of H2O

Chemical formulas are like shorthand notations for the composition of molecules, telling us precisely how many atoms of each element are present in a compound. One of the simplest yet most important compounds we encounter in our daily lives is water, with the chemical formula H2O. This formula indicates that each molecule of water contains two hydrogen atoms and one oxygen atom. Understanding how to break down these formulas is essential for mastering chemistry, whether you're in high school or delving into more advanced studies.
What Does H2O Mean?
The chemical formula H2O is more than just letters and numbers; it's a compact representation of how atoms are bonded in a molecule. Here's how you can interpret it:
- H: Represents hydrogen, an element that's symbolized by the first letter of its name.
- 2: Subscript number that tells us there are two hydrogen atoms in the molecule.
- O: Represents oxygen, with no subscript indicating one atom of oxygen.
Counting Atoms in H2O

Let's count the atoms in a molecule of water:
- Hydrogen atoms: 2 (since the subscript after hydrogen is 2).
- Oxygen atoms: 1 (no subscript, therefore, it's one atom by default).
Thus, in one molecule of water, there are a total of 3 atoms: 2 hydrogen and 1 oxygen.
Why is Counting Atoms Important?

Understanding how to count atoms in a chemical formula is crucial for several reasons:
- Chemical Reactions: When predicting the outcomes of chemical reactions, knowing the atom count helps in balancing equations.
- Stoichiometry: It's the basis for stoichiometric calculations, which involve determining the amount of substances involved in reactions.
- Chemical Nomenclature: Proper atom counting ensures accurate naming of compounds, avoiding confusion in chemical communication.
- Educational Insights: It provides a fundamental understanding of molecular structure and the concept of mole in chemistry.
Table: Examples of Other Simple Compounds
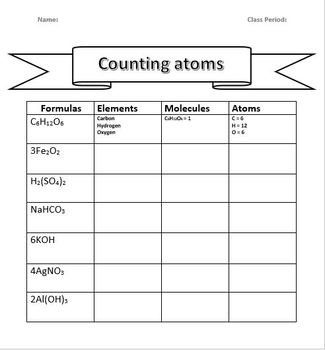
| Compound | Formula | Atom Count |
|---|---|---|
| Carbon Dioxide | CO2 | 1 Carbon, 2 Oxygen |
| Hydrogen Chloride | HCl | 1 Hydrogen, 1 Chlorine |
| Ammonia | NH3 | 1 Nitrogen, 3 Hydrogen |

🔍 Note: The lack of a number following an element symbol in a chemical formula implies there's only one atom of that element.
Key Takeaways in Counting Atoms in H2O

By dissecting the chemical formula of water, we've learned how to count atoms, which is the first step in understanding chemical compounds:
- Identify each element by its chemical symbol.
- Count the atoms for each element by noting the subscript number or lack thereof.
- Sum up the atoms to get the total count for the molecule.
These steps are not just useful for understanding water but for all chemical compounds, fostering a deeper comprehension of the molecular world.
The Bigger Picture

Now, we can appreciate the beauty of chemistry a bit more. Each molecule of water that we drink, use in cooking, or feel on our skin is made up of precisely two hydrogen atoms and one oxygen atom. This balance is what gives water its unique properties, allowing life as we know it to thrive. From the simplicity of H2O, we see the complexity of life, chemistry, and the universe.
What is the difference between an element and a compound?

+
An element is a pure substance consisting of only one type of atom, whereas a compound is made up of two or more different elements chemically bonded together in fixed ratios.
Can atoms in a compound be counted differently?

+
No, the atoms in a chemical formula are represented with subscripts indicating how many of each element are present. This is a standardized method of counting atoms in any chemical compound.
What makes water a universal solvent?

+
Water’s ability to dissolve many substances is due to its polar nature, where the hydrogen atoms have a slight positive charge and the oxygen atom has a slight negative charge, allowing it to interact and dissolve various compounds through hydrogen bonding.
Why is the study of water important in chemistry?

+
Water is not only vital for life but also plays a significant role in chemical reactions, temperature regulation, and as a medium for biological processes. Its study helps understand chemical properties, bonding, and its role in the ecosystem.



